

21 февраля исполнилось 90 лет члену-корреспонденту Российской академии наук, заслуженному деятелю науки РСФСР, советнику РАН, заместителю директора Института катализа СО РАН с 1961 по 1995 гг., профессору Роману Алексеевичу Буянову.
По окончании инженерного физико-химического факультета Московского химико-технологического института имени Д.И. Менделеева в 1950 г. Роман Алексеевич был направлен на Чирчикский электрохимический комбинат для участия в строительстве крупного промышленного объекта по выделению дейтерия методом ректификации жидкого водорода. Прошел путь от стажера до технического руководителя уникального, первого в мире производства тяжелого водорода. Под его непосредственным руководством строились и другие крупные промышленные объекты, такие как завод сухого льда, ТЭЦ, цех крепкой азотной кислоты.
В 1958 г. Р.А. Буянов перешел на работу в Международный Объединенный институт ядерных исследований (ОИЯИ) в г. Дубна. Здесь он занимался разработкой и промышленным освоением серийного водородно-гелиевого ожижителя, катализом при низких температурах, созданием сверхпроводящего соленоида и др.
В 1960 г. за работы в области химической технологии (разработка и промышленное освоение технологии получения дейтерия методом ректификации жидкого водорода) Р.А. Буянову присвоено звание Лауреата Ленинской премии.
В 1961 г. он защитил кандидатскую диссертацию и был приглашен Г.К. Боресковым в Институт катализа на должность его заместителя.
С августа 1961 г. Р.А. Буянов совмещал три должности: заместителя директора по науке, главного инженера и заведующего лабораторией. До 1964 г. руководил строительством Института, организацией его инфраструктуры и всех его служб. Одновременно принимал участие в решении вопросов, связанных с организацией СО АН СССР.
В 1967 г. за участие в создании Сибирского отделения АН СССР и развитие науки в Сибири награжден орденом Трудового Красного знамени.
В 1972 г. защитил докторскую диссертацию. В 1976 г. ему присвоено звание профессора, а в 1977 г. – почетное звание Заслуженный деятель науки РСФСР.
В 1979 г. Р.А. Буянов назначен руководителем Координационного центра стран СЭВ по проблеме “Разработка новых катализаторов и улучшение качества катализаторов, применяемых в промышленности”. С 1984 г. являлся представителем СССР в Совете уполномоченных стран СЭВ по этой проблеме.
В 1981 году избран членом-корреспондентом АН СССР. В 1982 г. награжден вторым орденом Трудового Красного знамени, в 1987 г. – орденом Октябрьской революции.
В 1996 г. Роман Алексеевич перешел на должность советника РАН и по-прежнему активно участвует в решении научных задач лаборатории дегидрирования, является членом научных и ученых советов, редакций научных журналов, координатором научных программ.
Под руководством Р.А. Буянова и с его участием разработана теория кристаллизации малорастворимых гидроксидов по механизму ориентированного наращивания и развита теория образования и эволюции их полиядерных гидроксокомплексов.
Развита фундаментальная теория магнитного действия катализаторов в низкотемпературной конверсии орто-водорода в пара-водород. Эти работы завершились созданием промышленного производства жидкого пара-водорода – ракетного топлива, на котором совершен полет космического корабля “Буран”.
Им с сотрудниками расшифрован “механизм карбидного цикла” в процессе каталитического разложения углеводородов с образованием углеродных нанонитей и волокон, разработана фундаментальная теория радикально-цепных неразветвленных реакций с участием гетерогенных катализаторов, проведен цикл исследований по механохимическим и термохимическим методам активации и повышению реакционной способности твердых реагентов в производстве катализаторов. Это позволило создать принципиально новые типы катализаторов высокоселективного гидрирования ацетиленовых и диеновых углеводородов и др.
Р.А. Буянов дал определение и очертил границы области науки, получившей название “Научные основы приготовления и технологии катализаторов”. Разработана научная классификация всех возможных причин дезактивации катализаторов.
В 60-70-х годах в содружестве с головным Институтом мономеров синтетического каучука (Ярославль) разработаны и внедрены в промышленность катализаторы для получения основных мономеров синтетического каучука (бутадиена, изопрена, стирола). На этих катализаторах вся промышленность СССР по производству синтетического каучука работала более 10 лет.
Под руководством Р.А. Буянова разработан и ряд других катализаторов и носителей, в том числе катализатор выделения серы по методу Клауса (лицензия продана французской фирме Рон-Пуленк и по ней во Франции построен цех), микросферический оксид алюминия для катализатора производства хлорвинила и др.
Р.А. Буянов – автор более 600 научных работ, монографий, обзоров, патентов. Под его руководством организованы и регулярно проводятся, начиная с 1983 г., Российские конференции по научным основам приготовления и дезактивации катализаторов.
Р.А. Буянов является главой школы в области научных основ приготовления катализаторов, в числе его учеников 6 докторов и 23 кандидата наук, успешно работающих в науке и промышленности. В настоящее время Роман Алексеевич активно участвует в выполнении ряда научных проектов, связанных с созданием новых технологий.
Научный совет по катализу ОХНМ РАН и редакция Каталитического бюллетеня сердечно поздравляют Романа Алексеевича с юбилеем, желают ему крепкого здоровья и новых творческих успехов!
 |
Российская академия наук Oтделение химии и наук о материалах НАУЧНЫЙ СОВЕТ ПО КАТАЛИЗУ |
Секретариат Научного совета по катализу ОХНМ РАН (НСК) предлагает Вашему вниманию сводный отчет о деятельности Совета и научных исследованиях в области катализа, выполненных научными коллективами под руководством членов Научного совета по катализу.
Отчет состоит из трех разделов:
Тексты отчетов, полученные от членов НСК и научно-исследовательских коллективов, практически не подвергнуты корректировке.
В 2016 году в рамках научно-организационной деятельности Научного совета по катализу ОХНМ РАН (НСК) были выполнены следующие мероприятия.
Изданы материалы проведенных конференций.
Секретариат НСК ведет переписку и текущую работу с членами Научного совета по катализу ОХНМ РАН.
Продолжается сотрудничество с организациями Академий наук РФ и стран СНГ, Министерствами РФ, институтами разных ведомств и другими организациями России, дальнего и ближнего зарубежья по различным вопросам научной, научно-организационной, учебно-преподавательской и общественной деятельности в области катализа.
Фундаментальные исследования в области создания новых каталитических систем
и применения физических методов для их диагностики
Исследование механизма конверсии ДМЭ в низшие олефины
Методами высокотемпературной ИК спектроскопии диффузного отражения in situ (ИКДО) в сочетании с квантово-химическим моделированием показано, что в процессе формирования активных центров превращения ДМЭ на поверхности цеолитных катализаторов на основе ZSM-5 принимают участие различные по силе кислотные центры Бренстеда (БКЦ). Слабые БКЦ участвуют в метоксилировании поверхности, сильные БКЦ протонируют ДМЭ. Взаимодействие метокси-группы с протонированным ДМЭ приводит к образованию кетена и формированию карбкатиона СН3+, который, атакуя ДМЭ, создает первую С–С связь через интермедиаты: карбониевые катионы и илидные частицы. Таким образом, образование олефинов при температурах 300–350°C происходит по карбоний-илидному механизму. Все типы БКЦ, интермедиаты и продукты (олефины и алканы) идентифицированы на поверхности катализаторов методом ИКДО, их структура подтверждена квантово-химическими расчетами.
академик С.Н. Хаджиев, д.х.н. Н.В. Колесниченко, д.х.н. Г.Н. Бондаренко
Институт нефтехимического синтеза им. А.В. Топчиева РАН, г. Москва
Исследование каталитической активности синтезированных наноразмерных суспензий в синтезе Фишера-Тропша в условиях трехфазной системы газ-жидкость-твердое тело
Показана возможность регулирования размера частиц дисперсной фазы Fe- и Со-катализаторов синтеза Фишера-Тропша путем варьирования таких параметров, как условия их приготовления, состав, природа и концентрация активной фазы и промотирующих добавок. Показано, что снижение размера части дисперсной фазы во всех случаях приводит к повышению производительности систем в отношении образования целевых продуктов синтеза. Оптимизация состава и условий приготовления контактов позволила увеличить производительность Со-катализаторов с 51 до 222 г/кг Со/ч, а железосодержащих суспензий – от 156 до 954 г/кг Fe/ч.
академик С.Н. Хаджиев, к.х.н. О.С. Дементьева
Институт
нефтехимического синтеза им. А.В. Топчиева РАН, г. Москва
Никель-кобальтовые катализаторы для кислородной и углекислотной конверсии метана в синтез-газ
Разработаны новые никель-кобальтовые нанесенные катализаторы для кислородной и углекислотной конверсии метана в синтез-газ, при 800-920°C показавшие конверсию метана 94-98%, выходы СО и Н2 91-98%, что является результатами, соответствующими мировому уровню. Сущность разработки – использование в качестве носителя для приговления катализатора цеолита структуры MFI, синтезированного ускоренным методом гидротермально-микроволнового синтеза. Использование данного носителя и оптимального сочетания активных компонентов позволяет предотвратить закоксовывание катализаторов. Катализаторы предполагается использовать для реализации инновационных процессов получения синтез-газа кислородной и углекислотной конверсией метана.
академик А.Г. Дедов, академик И.И. Моисеев, д.х.н., проф.
А.С. Локтев, аспирант И.Е. Мухин
Российский государственный университет нефти и газа им. И.М. Губкина, г. Москва
Катализаторы для конверсии пропан-бутановой фракции, изобутанола и гидроконверсии рапсового масла
Разработаны новые катализаторы для конверсии пропан-бутановой фракции, изобутанола и гидроконверсии рапсового масла, позволяющие получать ценные продукты нефтехимии – ароматические углеводороды или олефины С2-С4 c выходом более 50%. Сущность и новизна разработки – использование для приготовления катализаторов цеолитов структуры MFI и микромезопористых материалов, полученных ускоренным методом гидротермально-микроволнового синтеза. Микроволновое воздействие позволяет сократить продолжительность синтеза цеолитов и микромезопористых материалов, повысить их степени кристалличности. Значимость разработки обусловлена тем, что катализаторы, получаемые из синтезированных микроволновым методом цеолитов и микромезопористых материалов, превосходят аналоги, полученные традиционным гидротермальным методом.
академик
А.Г. Дедов, академик И.И. Моисеев, д.х.н., проф.
А.С. Локтев, к.х.н. Д.А. Левченко,
аспиранты А.А. Караваев, Е.А. Исаева
Российский государственный университет нефти и
газа им. И.М. Губкина, г. Москва
Полимер-иммобилизованные катализаторы смешанного типа для селективного гидрирования алкеновых и ацетиленовых соединений
Разработаны два подхода к синтезу моно- и биметаллических полимер-иммобилизованных катализаторов смешанного типа на основе Pd, Pd-Cu, Pd-Ag, закрепленных на поверхности неорганических носителей (SiO2 Al2O3, ZnO): модификация поверхности неорганического носителя ZnO полиэтиленгликолем или пектином и последующее закрепление комплексов палладия; фронтальная полимеризация металлосодержащего мономера в присутствии неорганического носителя. Показано, что получаемые органо-неорганические гибридные нанокомпозиты являются селективными катализаторами в реакции гидрирования алкеновых и ацетиленовых соединений. Модификация полимером способствует формированию высокодисперсных частиц активной фазы (2-4 нм) и их стабилизации; не происходит субстратной изомеризации при гидрировании аллилового спирта; обеспечивается селективное гидрирование ацетиленовых спиртов в олефиновые (полупродукты синтеза витаминов А, Е, К) и удаление ацетиленовых примесей при синтезе мономеров винильного типа из нефтяного сырья(ацетиленовых соединений из этилена, пропилена и др., ингибирующих их каталитическую полимеризацию).
академик
С.М. Алдошин, Н.Д. Голубева, Г.И. Джардималиева
Институт проблем химической физики РАН, г. Черноголовка
Фотокаталитическая технология очистки воздуха от паров фторированных анестетиков и других галогенсодержащих летучих загрязнителей в медицине
Совместно с Московским областным научно-исследовательским институтом акушерства и гинекологии изучена возможность применения фотокатализа для очистки воздуха от примеси севофлурана (CF3)2-CH-O-CH2F) – препарата для общей анестезии. Методами масс-спектрального и химического анализов установлено, что продуктами фотокаталитического окисления севофлурана в воздухе являются углекислый газ и фторид водорода. Показано, что выделяющийся в результате реакции HF может быть полностью связан химическим поглотителем из гранулированного карбоната кальция, установленным на выходе фотокаталитической ячейки. На основании полученных результатов предложена рабочая схема опытного устройства для очистки воздуха от паров севофлурана и других галогенсодержащих органических загрязнителей.
академик
С.М. Алдошин, И.Л. Балихин, В.И. Берестенко, И.А. Домашнев,
Е.Н. Кабачков, Е.Н. Куркин, В.Н. Троицкий, В.М.
Мартыненко, Е.Ю. Упрямова
Институт проблем химической физики РАН, г. Черноголовка
Катализаторы селективного гидрирования фенилацетилена на основе никеля, нанесенного на наноалмаз
С целью оптимизации состава активного центра катализатора селективного гидрирования в данной работе с использованием синхротронного рентгеновского излучения методами термопрограммированного восстановления in situ и EXAFS исследованы никелевые катализаторы, нанесенные на наноалмаз (ND), полученный методом детонационного синтеза. Проведено сравнение с данными для соединений, моделирующих возможные состояние никеля в активном центре. Использование для обработки данных EXAFS малораспространенного метода вейвлет-преобразования вместо общепринятого Фурье-преобразования позволило получить 3D-изображения, представляющие зависимость EXAFS-функции от расстояния до центрального атома и значений волнового вектора, и выявить два типа связывания никеля с поверхностью наноалмаза (через кислородсодержащие функциональные группы или непосредственно с углеродом поверхности). Показана возможность регулирования селективности нанесенного на ND никеля путем удаления одного из двух типов активных центров, прогреванием в аргоне при 900°С или окислением на воздухе при 300°С. Полученные фундаментальные данные позволили эффективно регулировать селективность катализаторов в гидрировании фенилацетилена.
д.х.н.
Е.С. Локтева, к.х.н. Е.В. Голубина, к.х.н. Я.В. Зубавичус и др.
Московский государственный университет имени М.В. Ломоносова,
Химический факультет, г. Москва
НИЦ Курчатовский институт, г.
Москва
Жидкофазное эпоксидирование пропилена и циклогексена с использованием органокатализатора
Методами УФ-, ИК- и ЯМР-спектроскопии подтверждена структура синтезированного органокатализатора, который представляет собой трифторацетофенон, ковалентно иммобилизованный на поверхности силиката SBA-15; оценена частота оборотов реакции (TOF) жидкофазного эпоксидирования пропилена и циклогексена, значительно превышающая соответствующие величины, полученные на других каталитических системах.
Установлена зависимость скорости жидкофазного эпоксидирования гексена пероксидом водорода на двух образцах титаносиликата, различающихся на два порядка размером частиц – 3 и 300 нм; в предположении протекания реакции на малых кристаллах в кинетической области определена степень диффузионного торможения на крупных кристаллах и проведена оценка величин эффективного коэфициента диффузии.
д.х.н.
Б.В. Романовский
Московский
государственный университет имени М.В. Ломоносова,
Химический факультет, г. Москва
Комплексообразование титана- и цирконадигидрофурановых металлациклов с алюминийорганическими соединениями и каталитическая активность образующихся комплексов в полимеризации ε-капролактона
Взаимодействием
титанадигидрофурановых и цирконадигидрофуранового металлациклов
Cp2Ti[η2-C(SiMe3)=C(Ph)–CR1(R2)O]
(где R1
= R2
= Me
(1) и R1
= H,
R2
= Ph)
и Cp2Zr[η2-C(SiMe3)=C(SiMe3)–С(Me)2O]
с iBu2AlH
синтезированы цвиттерионные 1:1 комплексы, содержащие мостик Al‐H–M (M= Ti,
Zr)
наряду с координационной связью Al–O.
В противоположность этому, реакция 1
с EtAlCl2
приводит к образованию 1:1 аддукта, содержащего только
координационную Al–O-связь.
По данным РСА, в результате комплексообразования с кислотой Льюиса
происходит сильное удлинение связей M–C(SiMe3)
и/или M–O
металлацикла, вследствие чего все полученные цвиттерионы оказваются
способными катализировать полимеризацию
ε-капролактона(ε-КЛ) с раскрытием цикла. Наиболее активен в полимеризации цвиттерионный
титановый комплекс, полученный из металлацикла 1. В этом случае
процесс полимеризации протекает уже при комнатной температуре, давая
через сутки поли-ε-КЛ
с выходом 72% и молекулярной массой 168000 (Mw/Mn
= 1.81). А если температуру повысить до 75°C, то уже через
один час молекулярная масса полимера достигает 195000 (Mw/Mn
= 2.14) при выходе 63%.
д.х.н.
В.Б. Шур, В.В. Бурлаков, В.С. Богданов, О.О. Соколова, П.
Арндт, А. Шпанненберг, М.Х. Миначева, К.А. Лысенко, И.А. Ананьев,
У. Розенталь
Институт элементоорганических соединений им. А.Н. Несмеянова РАН, г.
Москва
Институт
катализа им. Лейбница при Университете Ростока, г. Росток, Германия
Изучение морфологии катализатора, образующегося в вакууме на поверхности графита при высокотемпературном разложении HAuCl4 и Ni(NO3)2
Впервые на уровне единичных наночастиц выявлена морфология катализатора, образующегося в вакууме на поверхности графита при высокотемпературном разложении HAuCl4 и Ni(NO3)2, и определены функции его компонентов. Установлено, что катализатор состоит из смеси наночастиц золота и окисленного никеля. Каталитические свойства золото-никелевого покрытия определены по отношению к кислороду и водороду. При температуре 300°C на наночастицах в результате последовательной выдержки в Н2, а затем в О2 (двухэтапного процесса) образуются вода и ОН-группы, в то время как для катализатора, состоящего из наночастиц золота, синтез воды возможен только в результате последовательной экспозиции в Н2, затем в О2 и снова в Н2 (трехэтапного процесса). Показано, что наблюдаемые эффекты интерпретируются в рамках модели, предполагающей существование нескольких форм адсорбции водорода с существенно разной энергией связи. Сильно связанный водород локализован на золотых наночастицах и/или на интерфейсе золото-графит; водород, имеющий достаточно низкий барьер поверхностной миграции и способный к реакции с кислородом, адсорбирован на наночастицах окисленного никеля или на их интерфейсе с золотом. На покрытии, сформированном только из золотых наночастиц, слабо связанный водород, образовавшийся при первой экспозиции в H2, не сохраняется в достаточной концентрации к моменту начала экспозиции в кислороде, но вновь получается в достаточном количестве на третьей стадии эксперимента при повторной экспозиции образца в водороде.
д.х.н.
Б.Р. Шуб
Институт химической физики им. Н.Н. Семенова РАН, г. Москва
Оксидная система Na2WO4-MnxOy/SiO2 как катализатор окислительной конденсации метана
Оксидная система Na2WO4-MnxOy/SiO2 является перспективным катализатором процесса окислительной конденсации метана (ОКМ). Ранее было показано, что для данной каталитической системы кинетика образования продуктов ОКМ (этана и этилена) может быть описана в рамках модели Марса-ван-Кревелена, предполагающей попеременное (циклическое) восстановление и реокисление активных центров катализатора. Также было установлено, что смешанный оксид Na2WO4-MnxOy/SiO2 содержит две формы реакционноспособного кислорода. Первая является “сильно связанной” и может быть обратимо удалена при восстановлении катализатора в токе водорода и метана при температурах выше 600°C. Вторая – более слабо связанная; она обратимо удаляется из образца в режиме термопрограммированной десорбции при температурах выше 650°C.
Методом дифференциальной сканирующей калориметрии были экспериментально определены величины энергии связи решеточного кислорода в смешанном оксиде Na2WO4-MnxOy/SiO2. Установлено, что для “слабосвязанной” формы кислорода (содержание в системе около 56 мкмоль/г) она составляет ~337 кДж/моль. Эта величина указывает на то, что термодесорбция кислорода обусловлена переходом Mn(4+) → Mn(3+). Именно эта форма вносит наиболее существенный вклад в суммарную скорость стационарной каталитической реакции ОКМ. Количество (~ 443 мкмоль/г) и энергия связи кислорода более прочно связанного с оксидной решеткой типа (420-435 кДж/моль) указывают на то, что он ассоциирован с присутствующим в образце вольфрамом; однако на основании полученных данных сделать вывод о том, каким именно переходам и изменениям структуры отвечают удаление и присоединение кислорода в этой форме, не представляется возможным без проведения дополнительных исследований с привлечением структурно-чувствительных физических методов. С уверенностью, однако, можно констатировать, что величина энергии связи для этой формы кислорода столь велика, что в условиях стационарного катализа (т.е. в присутствии O2 в газовой фазе даже в небольших концентрациях) степень его вовлечения в каталитический процесс ОКМ не может быть существенной.
д.х.н.
М.Ю. Синев
Институт химической физики им. Н.Н. Семенова РАН,
г. Москва
Исследование селективного фотокаталитического окисления ароматических аминов
Открытая ранее реакция селективного фотокаталитического окисления ароматических аминов до п-аминофенолов синглетным кислородом была успешно распространена в 2016 году на соответствующие субстраты нафталинового и антраценового рядов. п-Аминонафтолы образуются, как и аминофенолы, с селективностью более 90%, характеристики процесса окисления 1-аминоантрацена устанавливаются. Получены данные по влиянию структуры фталоцианинового фотосенсибилизатора (природы центрального атома металла и заместителей) и его концентрации в растворе или на поверхности носителя на характеристики процесса окисления. Показано критическое значение содержания воды в реакционной среде – в отсутствие воды реакция не протекает. На основе этих и других изученных закономерностей предложены стехиометрия и схема механизма реакции, не противоречащая экспериментальным наблюдениям.
д.х.н. О.Л. Калия, к.х.н. Т.М. Федорова
Государственный научный центр “НИОПИК”, г. Москва
Катализаторы для процесса селективной гидроочистки бензинов каталитического крекинга
Продолжаются исследования и разработки триметаллических сульфидных катализаторов состава KNiWS и KCoMoS, в том числе с пониженным содержанием металлов, для процесса селективной гидроочистки бензинов каталитического крекинга с сохранением октанового числа. Показано, что щелочной металл изменяет не только свойства, но и концентрацию обоих типов (ГДС и ГИДО) активных центров KCoMoS2 или KNiWS2 частиц по схожему механизму. Установлено, что химические свойства бензина каталитического крекинга обуславливают состав используемой композиции: для сырья с высоким содержанием серы оптимальными являются NiWS системы, в то время как для сырья с низким количеством серы и высокой концентрацией олефинов подходят системы KCoMoS.
д.х.н.
П.А. Никульшин, к.х.н. А.В. Можаев, к.х.н. Д.И. Ишутенко, асп.
Ю.В. Анашкин, д.х.н. А.А. Пимерзин
Самарский государственный технический университет, г. Самара
Разработка фундаментальных основ аддитивной технологии переработки биомассы с целью получения ценных химических соединений и топливных компонентов
Разработаны методики синтеза наночастиц магнетита в порах сверхсшитого полистирола (СПС) марок MN270 (без функциональных групп), MN100 (функционализированный аминогруппами) и MN500 (функционализированный сульфогруппами). Установлена термостабильность СПС различных марок. Стабильные матрицы СПС MN270 и MN100 были использованы в дальнейшем для синтеза магнитных наночастиц оксида железа и Ru-содержащих магнитноотделяемых катализаторов. Определены оптимальные условия внедрения (метод импрегнации) прекурсора, его природа (железа (III) нитрат), его оптимальная концентрация (200 г/л в этаноле) и температура разложения (300 °С). Методами ПЭМ исследованы параметры частиц магнетита, образованных в порах СПС в результате термического разложения нитрата железа. Данные просвечивающей электронной микроскопии показали, что размер наночастиц оксида железа составляет 3,6±0,5 нм и не зависит от типа исходного СПС. Структура окиси железа была определена с помощью метода дифракции рентгеновских лучей.
Исследованы магнитные свойства синтезированных Ru-содержащих магнитноотделяемых катализаторов. Методом РФЭС определён состав их поверхности. Было обнаружено, что в ходе синтеза наночастиц оксида железа образуются исключительно магнетитовые наночастицы. ИК-спектроскопическое исследование показало, что условия восстановления катализаторов не оказывают влияния на полимерную матрицу СПС, а адсорбция глюкозы (субстрата) и сорбита (продукта реакции гидрирования) на катализаторах незначительна.
Проведено тестирование синтезированных Ru-содержащих магнитноотделяемых катализаторов в реакции гидрирования глюкозы до сорбита. Определены оптимальные условия реакции (температура, парциальное давление водорода, концентрации субстрата и катализатора, интенсивность перемешивания) и стабильность катализаторов в нескольких циклах использования. Наиболее эффективными оказались образцы катализаторов на основе СПС марки MN100, приготовленные с использованием в качестве прекурсора рутения (IV) гидроксотрихлорида. Стабильность всех образцов синтезированных катализаторов в гидротермальных условиях процесса гидрирования глюкозы была подтверждена трехкратным использованием без заметного снижения их активности.
д.х.н.,
проф. Э.М. Сульман
Тверской
государственный технический университет, Институт нано- и биотехнологий, г. Тверь
Эффективный нанокатализатор для окислительной функционализации ароматических углеводородов
Разработан новый эффективный гетерогенный нанокатализатор окислительной функционализации ароматических углеводородов на основе NiIII-комплексов, допированных в силикатные наночастицы ([(bpy)xNiIII]@SiO2. Преимущества этого нанокатализатора по сравнению с известными гомогенными молекулярными никелевыми и палладиевыми катализаторами заключаются в высокой активности, количественных выходах продуктов фторалкилирования, десятикратном снижении рабочей концентрации катализатора (менее 1%), стабильности во времени и легкой регенерации.
д.х.н.Ю.Г. Будникова,
д.х.н. А.Р. Мустафина, к.х.н. М.Н. Хризанфоров,
к.х.н. С.В. Федоренко, асп. С.О. Стрекалова, к.х.н. К.В. Холин,
М.Е. Жилкин
Институт органической и физической химии им. А.Е. Арбузова КазНЦ РАН, г. Казань
Контроль скорости разложения фосфорорганических ингибиторов ацетилхолинэстеразы с помощью биомиметических катализаторов
Предложены новые биомиметические катализаторы (пиримидинсодержащие и фосфониевые ПАВ), позволяющие контролировать скорость разложения фосфорорганических ингибиторов ацетилхолинэстеразы (от ускорения до аномального ингибирования), проявляющие высокую эффективность в низких концентрациях и выраженную субстратную специфичность.
академик
О.Г. Синяшин, д.х.н., проф. Л.Я. Захарова, проф. В.С. Резник,
д.х.н. В.Э. Семенов,
к.х.н. Д.Р. Габдрахманов, к.х.н. Г.А.
Гайнанова, к.х.н. Ф.Г. Валеева, асп. Д.А. Самаркина
Институт
органической и физической химии им. А.Е. Арбузова КазНЦ РАН, г.
Казань
Конформационый анализ заряженных бис хелатных комплексов Ni(II)
Решена задача полного конформационного анализа заряженных бис хелатных комплексов Ni(II) на основе P,N-содержащих гетероциклических лигандов (1,5-диаза-3,7-дифосфациклооктанов) – наиболее активных никелевых катализаторов электрохимического получения водорода. Обнаружены корреляции структурно-динамических параметров этих комплексов с их каталитической активностью. Максимальной активности катализатора соответствует высокая конформационная лабильность как гетероциклического фрагмента, так и геометрии центрального иона, реализующихся со стерически незагруженными заместителями на фосфоре и ароматическими группами на азоте.
академик
О.Г. Синяшин, д.х.н., проф. А.А. Карасик, д.х.н. Ш.К. Латыпов,
А.Г. Стрельник, к.х.н. А.С. Балуева, Ю.С. Спиридонова
Институт органической и физической химии
им. А.Е. Арбузова КазНЦ РАН, г. Казань
Региоселективное амидирование бинора-S органическими нитрилами в присутствии воды, катализированное FeCl3·6H2O
Впервые осуществлено однореакторное амидирование бинора-S органическими нитрилами (ацетонитрил, пропионитрил, валеронитрил, бензонитрил) под действием FeCl3·6H2O в условиях: 150°C, 6 ч. Реакция проходит нетривиально путем региоизбирательного разрыва связи С4-С5 циклопропанового кольца бинора-S с образованием соответствующих N-замещенных амидов с выходами 70-90%. Омылением амидов синтезирован 10-амино-гексацикло[12.1.0.0.2,60.3,80.5,709,13]тетрадекан (аналог известного противовирусного препарата 1-аминоадамантана – мидантана), представляющий практический интерес для производства ноотропных и противовирусных препаратов.
проф.
Р.И. Хуснутдинов, к.х.н. Т.М. Егорова
Институт
нефтехимии и катализа РАН, г. Уфа
Короткий и эффективный путь стереоселективного каталитического синтеза природного лембехина В – индуктора раннего апоптоза в клетках лейкемии
Разработан эффективный путь стереоселективного синтеза лембехина B с применением на ключевой стадии новой реакции Ti-катализируемого кросс-цикломагнирования О-содержащих и алифатических 1,2-диенов с помощью реактивов Гриньяра. В результате реакции цикломагнирования 1,2-нонадекадиена и тетрагидропиранового эфира 13,14-пентадекадиенола с помощью EtMgBr в присутствии металлического Mg и катализатора Cp2TiCl2 (10 мол %) в условиях: 20-22°C, 7 ч, Et2O, после гидролиза реакционной массы получен тетрагидропирановый эфир (13Z,17Z)-тетраконта-13,17-диенола с выходом 88%. В результате последовательных трансформаций синтезированного эфира получен целевой лембехин В.
С целью изучения закономерностей влияния структуры лембехина на проявляемую им биологическую активность с применением разработанного подхода удалось синтезировать линейку рацемических и природных лембехинов В, в том числе длинно- (С36-С40) и короткоцепочечных (С25-С34) аналогов. С привлечением проточной цитофлуорометрии впервые показано, что лембехин B является селективным индуктором раннего апоптоза клеточных культур лейкемии Jurkat, HL-60 и K562. В настоящее время проводятся интенсивные исследования в данном направлении с целью реализации стереоселективных методов синтеза всей линейки природных лембехинов и их аналогов, в том числе для проведения полномасштабных фармакологических исследований по изучению биологической активности и выявления закономерностей структура – противоопухолевая активность.
чл.-корр.
РАН У.М. Джемилев, д.х.н. В.А. Дьяконов,
к.х.н. А.А. Макаров,
д.м.н. Л.У. Джемилева
Институт нефтехимии и катализа РАН, г. Уфа
Ti-Катализируемое кросс-цикломагнирование 1,2-диенов в стереоселективном синтезе практически важных феромонов насекомых-вредителей
В продолжение исследований по изучению областей приложения разработанных в лаборатории новых фундаментальных реакций каталитического циклометаллирования непредельных соединений впервые показано, что реакции каталитического цикломагнирования 1,2-диенов являются удобным инструментом для стереоселективного построения соединений, содержащих в своей структуре 1Z,5Z-диеновые фрагменты, и могут быть с успехом использованы при разработке препаративных методов синтеза феромонов насекомых. Так, с применением на ключевой стадии синтеза кросс-цикломагнирования алифатических 1,2-диенов с O-содержащими алленами, взятых в соотношении 1.2:1, в условиях (Et2O, 20-22°C, 4 ч) разработаны стереоселективные методы получения гексадека-7Z,11Z-диен-1-аля – компонента феромонов минирующей моли цитрусовых растений Phyllocnis tiscitrella, гексадека-7Z,11Z-диен-1-ил ацетата – аттрактанта розового коробочного червя хлопчатника Pectinophora gossypiella, пентакоза-7Z,11Z-диен и нонакоза-7Z,11Z-диена – аттрактантов плодовой мухи Drosophila melanogaster с высокими выходами (60-75%), представляющих исключительный интерес для сельского хозяйства для борьбы с вредителями сельскохозяйственных и декоративных растений.
чл.-корр.
РАН У.М. Джемилев, д.х.н. В.А. Дьяконов,
к.х.н. И.И. Исламов,
к.х.н. А.А. Макаров
Институт нефтехимии и катализа РАН, г. Уфа
Гетерогенно-каталитические способы синтеза практически важных пиридинов
Соединения с пиридиновым фрагментом играют ключевую роль в некоторых биологических процессах, наиболее важные из них – окислительно-восстановительные процессы с участием кофермента никотинамидадениндинуклеотида (NADP). На основе пиридина и алкилпиридинов синтезируют важные фармацевтические препараты, сельскохозяйственные химикаты, латексы, экстрагенты, ингибиторы коррозии металлов, растворители, поверхностно-активные вещества, ускорители вулканизации каучука и многое другое.
С целью разработки селективных гетерогенно-каталитических способов
синтеза практически важных N-гетероциклических соединений впервые
изучены каталитические свойства в синтезе пиридина и его
алкилпроизводных реакцией этанола с формальдегидом и аммиаком новых
каталитических систем, приготовленных на основе цеолитов Y
и MOR с комбинированной микро-мезо-макропористой структурой (Y
-ммм и MORммм) и аморфных мезопористых алюмосиликатов
(ASM) с различным мольным соотношением Si/Al = 40
и 80, отличающихся текстурой и кислотными свойствами. Установлена
высокая активность в указанной выше реакции каталитических систем на
основе цеолита H-Yммм и алюмосиликатов
д.х.н.
Б.И. Кутепов, д.х.н. Н.Г. Григорьева, к.х.н. Р.Р. Талипова,
к.х.н. М.Р. Аглиуллин,
к.х.н. С.В. Бубеннов, асп. Н.А. Филиппова,
асп. А.М. Гатаулин
Институт нефтехимии и катализа РАН, г. Уфа
Синтез новых полициклических молекул, имеющих девять конденсированных циклов в составе своей структуры “лестничного” типа
Разработан удобный способ синтеза новых полициклических молекул, имеющих девять конденсированных циклов в составе своей структуры “лестничного” типа и содержащих систему 5,11-дигидроиндоло[3,2-b]карбазола в качестве базового остова. Показана возможность модификации каркаса полученных конденсированных производных. Предложенный способ синтеза конденсированных производных 5,11-дигидроиндоло[3,2-b]карбазола основан на последовательности реакций ацилирования по Фриделю-Крафтсу и Pd-катализируемой С-Н активации, исходя из доступных соединений – 5,11-диалкил-6,12-ди(гетеро)арилзамещенных индоло[3,2-b]карбазолов. Впервые была показана возможность металл-катализируемой активации С-Н связи в молекулах 5,11-дигидроиндоло[3,2-b]карбазолов.
академик
В.Н. Чарушин, к.х.н. Г.Л. Русинов, к.х.н. Р.А. Иргашев, Н.А. Казин, Г.А. Ким
Институт органического синтеза им. И.Я. Постовского УрОРАН, г. Екатеринбург
Синтез нового класса несимметричных гетероаценов
Разработан эффективный способ получения производных 6H-бензо[4',5']тиено-[2',3':4,5]тиено[3,2-b]индола, нового класса несимметричных гетероаценов, а также их гексациклических аналогов, с использованием реакции индолизации по Фишеру в качестве ключевой стадии.
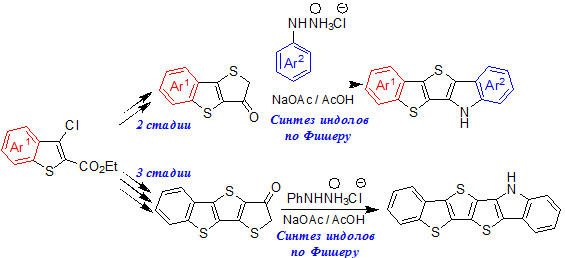
академик В.Н. Чарушин, к.х.н. Г.Л. Русинов, к.х.н. Р.А. Иргашев, А.А. Кармацкий, Г.А. Ким
Институт органического синтеза им. И.Я. Постовского УрОРАН, г. Екатеринбург
Исследование влияния цеолитной матрицы β-цеолита (модуль, кислотность, содержание цеолита, платины) на показатели реакции изомеризации н-гептана (выход суммы изомеров, выход ди- и триметилзамещенных изомеров гептана)
Приготовлены и исследованы в реакции изомеризации н-гептана Pt/BEA/Al2O3 катализаторы с варьированием содержания в катализаторе цеолита и платины, нанесенной из двух типов предшественников – гексахлорплатиновой кислоты и аммиаката платины. Установлено, что оптимальными катализаторами являются системы с 0,2-0,3 % мас. платины, нанесенной из водного раствора H2PtCl6, демонстрирующие близкий к равновесному выход метилциклопентана, а также высокие выходы изомеров гептанов при минимальном вкладе побочных реакций гидрокрекинга алканов. Катализаторы могут быть использованы в процессе гидроизомеризации С7-углеводородов в прямогонных бензиновых фракциях и в бензинах каталитического риформинга для улучшения их экологических характеристик.
д.х.н., проф. А.С. Белый, к.х.н. М.Д. Смоликов
Институт проблем переработки углеводородов СО РАН, г. Омск
Исследование этенолиза алкенов C5+ как метода синтеза пропилена
Изучена возможность синтезапропилена из смеси алкенов С5+иэтилена на катализаторах метатезиса, обладающих кислотной функцией. Посредством термодинамических расчетов показано, что превращение алкена С5+ в смеси с этиленом позволяет получать пропилен с высоким выходом (до 60% для пентена-1 при стехиометрическом соотношении с этиленом). Селективность процесса по пропилену при сверхстехиометрическом количестве этилена в сырьевой смеси может превышать 90%. По результатам испытаний установлено, что на катализаторе Re/ВA обеспечивается высокая селективность процесса (85-90% по пропилену), тогда как процесс на Re/SZA осложняется протеканием побочных реакций олигомеризации и метатезиса. Результаты могут быть использованы при создании новых технологий переработки газового сырья с получением продуктов для нефтехимии.
к.х.н. А.В. Лавренов, к.х.н. Е.А. Булучевский
Институт проблем переработки углеводородов СО РАН, г. Омск
Исследование влияния компонентного состава цеолитсодержащего катализатора на совместные превращения растительных масел с вакуумными газойлями различного группового состава
Исследовано влияние компонентного состава цеолитсодержащего катализатора на совместные превращения растительных масел с вакуумными газойлями различного группового состава. Определено, что при высоком содержании цеолита Y в составе катализатора (20,0 мас. %) выходы целевых продуктов крекинга смесевого сырья существенно не зависят от жирнокислотного состава растительного масла, входящего в его состав. Влияние типа масла проявляется только при повышении содержания в составе активного компонента катализатора цеолита ZSM-5. Показано, что многокомпонентная активная матрица (аморфный алюмосиликат + оксид алюминия + монтмориллонит) обеспечивает протекание первичного крекинга, способствует достижению высокой конверсии смесевого сырья и повышенному выходу бензиновой фракции. Кроме того, вышеуказанная матрица обеспечивает повышенное образование кокса, что имеет большое значение для замыкания теплового баланса при превращении глубоко гидроочищенных вакуумных газойлей. Результаты важны для разработки технологий переработки растительной биомассы в компоненты моторных топлив.
к.т.н. В.П. Доронин, к.х.н. П.В. Липин
Институт проблем переработки углеводородов СО РАН, г. Омск
Катализатор ароматизации газообразных углеводородов
Для процесса ароматизации низших алканов синтезированы элементоалюмосиликаты (Э-АС) структурного типа цеолита ZSM-5, содержащие в своем составе наряду с атомами кремния и алюминия атомы Ga, In, Zn и Zr. На основании данных структурно-морфологических исследований полученных образцов установлено, что введение металлов в цеолитную структуру приводит к образованию различных по морфологии и элементному составу частиц.
Исследования электронного состояния активных центров Э-АС показали, что катионы Ga и Zn связаны с ионами кислорода в каналах цеолита с энергиями связи, характерными для оксидов этих элементов. Высокое значение энергии связи Zn3d (1023.0 эВ) объясняет стабильность ионов цинка в структуре Zn-AC во время реакции. Изоморфное замещение ионов Si4+ в кристаллической решетке цеолита на ионы Zr4+ и In3+, несмотря на относительно низкие значения энергии связи Zr3d (183.4 эВ) и In3d (446.3 эВ), также обуславливает стабильность систем Zr-АС и In-AC. Лишь при сильном нагреве после разрушения канальной структуры цеолита наблюдается агрегирование Zr и In в оксидные кластеры. Полученные Э-АС проявляют высокую активность и селективность в процессе превращения пропана в ароматические углеводороды (АрУ), а наиболее эффективным является катализатор с добавкой галлия: селективность образования на нем целевого продукта превышает 52% при конверсии пропана 97%. Катализатор может использоваться для переработки попутных нефтяных и отходящих нефтезаводских газов в практически важные продукты.
д.х.н.
А.В. Восмериков
Институт химии нефти СО РАН, г. Томск
Применение автоклавных технологий для растворения оксидных матриц катализаторов; разработка катализаторов для изомеризации н-алканов
С целью разработки методики оперативного контроля структурно-химических изменений катализаторов нефтепереработки в процессе эксплуатации проводилась оценка эффективности применения автоклавных технологий для растворения оксидных матриц катализаторов и вскрытия различных соединений, химических фаз цветных и благородных металлов, входящих в состав катализаторов. Установлена эффективность данного метода при селективном выделении отдельных исследуемых фаз для повышения точности методов рентгеновской дифрактометрии. Предложена методика оценки размеров частиц платины в образцах катализатора риформинга, в том числе отработанного.
Проводятся исследования по разработке новых катализаторов для изомеризации н-алканов C4-C6 на основе диоксида циркония, модифицированного редкоземельными металлами, а также методов их синтеза путем легирования основной фазы анионными промоторами и катионами двух- и трехвалентных металлов (Y3+, Се3+, Sc3+, Fe3+, Mn2+, Са2+ и др.), стабилизирующими метастабильные модификации. Разработка нового семейства гетерогенных кислотных катализаторов на основе диоксида циркония является важным достижением науки в области катализа процессов нефтепереработки за последнее десятилетие, так как такие системы позволяют осуществлять изомеризацию н-парафинов при низкой температуре с высоким выходом высокоразветвленных изомеров.
д.т.н., проф. В.П. Твердохлебов
Сибирский Федеральный университет, Институт нефти и газа, г. Красноярск,
Институт химии и химической технологии СО РАН, г. Красноярск
Исследование механизма реакций Соногаширы и Хека с ангидридами ароматических кислот
Путем применения набора новых подходов, базирующихся на использовании феномена искусственной многомаршрутности, определены детали механизма элементарных стадий реакций сочетания Соногаширы и Хека с ангидридами ароматических кислот в качестве арилирующего реагента. В частности, установлена степени обратимости стадий с участием субстратов, а также быстрые и скорость определяющие стадии каталитических циклов.
В реакции Соногаширы результаты исследования зависимостей дифференциальной селективности реакций от различных параметров (отношения конкурирующих субстратов, природы и концентрации партнера по сочетанию) с одновременным анализом интегральных кинетических данных позволили установить, что арилгалогениды и арилацетилены участвуют в быстрых необратимых стадиях в присутствии палладиевых «безлигандных» каталитических систем. Скорость реакции определяется одной или комбинацией нескольких мономолекулярных стадий, в которых субстрат и партнер по сочетанию непосредственно не участвуют. Зависимость дифференциальной селективности по впервые обнаруженным полициклическим продуктам реакции Соногаширы указывает на участие основания в формировании катализатора, активного в образовании этих продуктов. Чувствительность дифференциальной селективности к природе предшественника катализатора согласуется с существованием нескольких (минимум двух) типов активных соединений, участвующих в образовании полициклических продуктов.
В реакции Хека с ароматическими ангидридами результаты исследования дифференциальной селективности в комбинации с гамметовскими корреляциями, полученных для конкурентных и неконкурентных вариантов реакции, а также результаты анализа интегральных кинетических данных расходования конкурирующих субстратов указывают на участие ангидрида и сочетающегося с ним алкена в быстрых необратимых стадиях каталитического цикла. Активность катализатора контролируется скоростью одной или нескольких мономолекулярных стадий каталитического цикла, в которых ангидрид и алкен непосредственно не участвуют.
д.х.н., проф. А.Ф. Шмидт
Иркутский государственный университет, г. Иркутск
Синтез катионных ацетилацетонатных комплексов палладия с анилиновыми лигандами
Впервые осуществлен синтез серии катионных ацетилацетонатных комплексов палладия с анилиновыми лигандами типа [(acac)Pd(L)2]BF4 (где L= NH2(p-Tol)2, NH2(о-Tol)2, NH2(2,6-Me2-Ph)2, NH2(2,6-i-Pr2-Ph)2). Методами РСА, ЯМР, ИКС и DFT-расчетов установлено образование водородной связи типа NH…FBF3 между анионом координированными анилиновыми и аминовыми лигандами, а также сохранение псевдокристаллической структуры в растворе. Обнаружено, что комплексы типа [(acac)Pd(L)2]BF4 (L= MeCN,NH2Ar) активны в индивидуальном виде, без добавок сокатализатора, в аддитивной полимеризации норборнена, димеризации стирола и его производных с селективностью до 90%. Предложен, экспериментально и теоретически обоснован механизм формирования постулируемых активных катионных гидридных комплексов палладия из прекурсора [Pd(acac)(MeCN)2]BF4 без участия сокатализатора на примере реакции димеризации стирола путем активации связи бор–фтор в анионе тетрафторбората, с последующей атакой образующегося трифторида бора κ2-O,O-ацетилацетонатного лиганда, связанного с палладием. Исследованы димеризация стирола и полимеризация норборнена в присутствии каталитических систем состава [(acac)Pd(L)2]BF4 и BF3·OEt2 (L= MeCN,NH2Ar,NHR2). Обнаружено, что в ряде случае данные каталитические проявляют высокую активность (до 5,5·106 гПНБ/{мольPd·ч}) в полимеризации норборнена, сопоставимую с лучшими представленными в научной литературе палладиевыми аналогами.
Получены новые конкурентоспособные каталитические системы для реакций полимеризации норборнена, 5-метоксикарбонилнорборнена, синтеза олигомеров норборнена с концевой винильной группой, сополимеризации норборнена с 5-метоксикарбонилнорборненом на основе систем состава {комплекс Ni(0)} /BF3OEt2, Ni(acac)2/PR3/AlEt3/HА/BF3·OEt2, [(acac)Pd(L)]BF4/BF3·OEt2, [(acac)Pd(L’)2]BF4/BF3·OEt2, [(acac)Pd(PPh3)(PR3)]BF4/BF3·OEt2, которые по активности сопоставимы с аналогами, представленными в патентной и научной литературе.
д.х.н., проф. Ф.К. Шмидт
Иркутский государственный университет, г. Иркутск
Теоретическое и экспериментальное изучение механизма катализа реакций нуклеофильного присоединения к ацетилену суперосновными системами MOH/DMSO
Методами квантовой химии и органического синтеза изучен механизм катализа нуклеофильного присоединения к ацетилену в суперосновных каталитических системах типа MOH/DMSO (М – щелочной металл). Развитие ранее предложенной модели катализа этими супероснованиями с участием недиссоциированных молекул КОН (NaOH) позволило идентифицировать первичные суперосновные каталитические комплексы, в которых молекулы DMSO входят в первую сольватационную сферу, и уточнить их последующее комплексообразование как с нуклеофилами, так и с ацетиленом. Показано, что первичные каталитические комплексы KOH•5DMSO и NaOH•4DMSO стабилизированы межлигандным взаимодействием. Результаты позволяют проводить оценку энергии активации реакций нуклеофильного присоединения воды, спиртов, сероводорода, тиолов и СН-кислот к тройной связи и качественно согласуются с экспериментом. Предложенная уточненная модель объясняет увеличение выхода продуктов этинилирования кетонов ацетиленом (нуклеофильное присоединение ацетиленид-иона к связи С=О) в присутствии небольших количеств воды, подавляющих енолизацию кетонов.
академик Б.А. Трофимов
Иркутский институт химии СО РАН им. А.Е. Фаворского СО РАН, г. Иркутск
проф. Н.М. Витковская, проф. В.Б. Кобычев, д.х.н. Е.Ю. Ларионова, В.Б. Орел
Иркутский государственный университет, г. Иркутск
Исследование процессов активации молекулярного водорода новыми перспективными каталитическими системами
Для гидрирования непредельных соединений в современной химической промышленности преимущественно используют катализаторы на основе переходных металлов. Стремление снизить стоимость катализаторов и их воздействие на окружающую среду определяет интерес к созданию новых каталитических систем для процессов гидрирования на основе более распространенных и безвредных элементов. В частности, среди систем на основе элементов главных групп значительный интерес представляют фрустрированные Льюисовские пары, для которых в последнее время продемонстрирована возможность их использования в процессах активации Н2 и гидрирования широкого круга непредельных соединений.
Показана возможность значительного усиления сигнала ЯМР за счет эффекта индуцированной параводородом поляризации ядер (ИППЯ) при активации параводорода с использованием ряда не содержащих атомов металла каталитических систем, представляющих собой фрустрированные Льюисовские пары анса-аминоборанов. Активация параводорода этими “молекулярными пинцетами” приводит к усилению сигналов в спектре ЯМР 1Н до 20 раз. Полученные результаты наглядно демонстрируют применимость метода ИППЯ для детального изучения механизма активации Н2 системами, не содержащими атомов металла. Все основные результаты получены впервые и являются существенным продвижением вперед в области развития методов значительного усиления сигнала ЯМР и применения этих методов для исследования механизмов важных каталитических процессов.
д.х.н. И.В. Коптюг
Международный томографический центр СО РАН,
г. Новосибирск
Полифункциональные нанороботы для синтеза наноразмерных алмазов
Открыто неизвестное ранее явление – физико-химический наноробот (ФХНР). Показано, что высокодисперсные частицы металлов подгруппы железа являются высокоактивными полифункциональными материалами (структурами), способными селективно выполнять ряд функций, инициировать протекание нескольких процессов разной природы и управлять ими в определенной последовательности технологических стадий. Такие полифункциональные наноразмерные структуры, выступающие в роли физико-химических нанороботов, управляют многостадийной технологией. Явление ФХНР впервые было обнаружено нами на примере синтеза углеродных наноструктур при разложении углеводородов на Ni-частицах по расшифрованному нами механизму карбидного цикла.
Разработан способ управления ФХНР с целью получения различных продуктов (материалов). В частности, предложен способ синтеза наноразмерных алмазов из углеводородов при атмосферном давлении и температуре ниже 700°C и другие синтезы.
чл.-корр. РАН Р.А. Буянов, академик В.Н. Пармон,
чл.-корр. РАН З.Р. Исмагилов, д.х.н. О.Ю. ПОдъячева
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Получение винилфенолов путем каталитической конденсации фенола с ацетальдегидом
Впервые обнаружена и исследована ранее неизвестная реакция фенола с ацетальдегидом, позволяющая в одну стадию получать о-винилфенол.
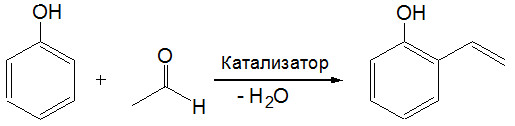
Показано, что лучшим является катализатор, представляющий собой оксид хрома, нанесенный на γ-Al2O3 и модифицированный солями калия. Катализатор позволяет получать о-винилфенол с селективностью близкой к 100% по фенолу и 87%-ной селективностью по ацетальдегиду. Основной побочный продукт реакции, кротоновый альдегид, также является ценным химическим продуктом. Винилфенолы используются при получении лекарств, косметики и в качестве консерванта в пищевой промышленности. Полимеры на основе винилфенолов находят применение в производстве фоточувствительных материалов, антибактериальных средств и жидких кристаллов.
д.х.н., проф. Г.И. Панов, д.х.н. А.С. Харитонов,
к.х.н. М.В. Парфенов,
к.х.н. Л.В. Пирютко, к.х.н. И.Е. Сошников, к.х.н. Е.В. Староконь
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Новый способ получения триэтаноламина улучшенного качества
Разработан способ получения триэтаноламина (ТЭА) улучшенного качества, который широко применяется в различных отраслях промышленности, в том числе в производстве биоцидов, полупродуктов в синтезе лекарственных препаратов и парфюмерно-косметических средств. Реакцию аммонолиза оксида этилена проводят при температуре 110-150°C и давлении 2-15 МПа при подаче оксида этилена с одной стороны и воды с аммиаком с другой стороны, при мольных отношениях: аммиак/оксид этилена 1-2, вода/аммиак 5-15. Отличительной особенностью способа является проведение процесса в микроканальном реакторе проточного типа, содержащем камеру смешения и микроканальные пластины с каналами вытянутой формы, причем реактор имеет два входа: один для подачи оксида этилена, другой для подачи водно-аммиачной смеси. Подачу осуществляют непрерывно на входе в смеситель с последующим равномерным смешением в микроканалах при временах контакта 0,1-10 минут. Микроканальные пластины выполнены из металла, устойчивого к водно-аммиачной среде, при этом каналы в микроканальной пластине могут быть параллельными или взаимопересекающимися и могут иметь глубину и ширину от 0,2 до 2 мм. Способ позволяет повысить качество получаемого продукта – улучшить показатель цветности, а также повысить конверсию и селективность процесса.
академик В.Н. Пармон, д.х.н. Л.Л. Макаршин, д.т.н. З.П. Пай, д.х.н. Е.Г.
Жижина,
к.х.н. Д.В. Андреев, к.х.н. А.Г. Грибовский, к.х.н. Е.Е.Сергеев, Д.Ю. Ющенко
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Разработка процессов каталитического окисления органических соединений с применением экологически безопасных окислителей для получения импортозамещающих продуктов
Показана возможность получения востребованных, в том числе импортозамещающих продуктов тонкого органического синтеза – биологически активных веществ или их предшественников. Реакция двухфазного окисления органических субстратов перексидом водорода в присутствии бифункциональных металлокомплексных катализаторов Q3{PO4[WO(O2)2]4 протекает в органической фазе через транспорт кислорода от пероксокомплекса к субстрату. Показано, что метод межфазного катализа позволяет получать N-оксиды фосфоновых кислот с селективностью 85% при 90% конверсии субстрата (используются в производстве гербицидов) и карбоновые кислоты с высокими выходами 86-97% (используются в пищевой, медицинской и других отраслях промышленности).
д.т.н. З.П. Пай, к.х.н. Л.В. Малышева, П.В. Бердникова, Д.Ю. Ющенко
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Растворы Mo-V-фосфорных гетерополикислот в катализе: от синтеза к применению
Разработка и масштабирование способа получения модифицированных высокованадиевых растворов ГПК, обладающих высокой термостойкостью и быстро регенерируемых, обеспечила возможность создания на их основе технологичных катализаторов окисления субстратов разных классов.
Для новых растворов ГПК установлены зависимости физико-химических свойств (ρ, η,Е, рН) от степени восстановления и температуры. Подтверждено, что изменения свойств катализаторов полностью обратимы.
Результаты исследования открывают хорошие перспективы разработки в присутствии растворов Мо-V-Р ГПК процессов получения важных органических продуктов, например, диалкилбензохинонов и замещенных антрахинонов.
д.х.н. Е.Г. Жижина, к.т.н. Л.Л. Гогин, Ю.А. Родикова, д.т.н. З.П. Пай
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Исследование проникающей способности аэрозольных наночастиц с различными химическими свойствами поверхности в органы лабораторных мышей методом ускорительной масс-спектрометрии
В исследованиях проникновения органических аэрозолей в живой организм впервые в мире продемонстрирован метод прямой регистрации частиц при крайне низкой удельной дозе воздействия – зафиксировано 90 нг частиц в 1 г органа мыши при радиоактивности 0.6 Бк/г, а также при их вдыхании в естественных условиях.
Для получения меченых аэрозолей разработаны методы синтеза из 14С-метанола 14С-стирола, из которого затем получили полистирольные микросферы (в одном латексе – 225 нм, в другом – 80 нм). Из меченых латексов получали аэрозоль с низкой концентрацией, которым дышали мыши в течение 30 минут 5 дней. Изотопный анализ проб органов мышей методом сверхчувствительной ускорительной масс-спектрометрии показал, что частицы размером 225 нанометров в очень низкой концентрации (103 шт/см3), проникая в организм через лёгкие, накапливаются в почках, печени и мозге и при этом не накапливаются в сердце. Частицы размером 80 нм с концентрацией 104 шт/см3 вдыхаемого воздуха остаются в лёгких минимум полгода после воздействия.
Е.В. Пархомчук, А.И. Таратайко, Д.Г. Гулевич, А.М. Бакланов,
П.Н. Калинкин, А.Г. Окунев, С.А. Растигеев, В.А. Резников, В.В. Пархомчук,
Т.А. Трубицына, Е.А. Прокопьева, Е.И. Соловьева, Д.В. Кулешов,М.А. Кулешова
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск,
НГУ, ИОХ СО РАН, ИХКиГ СО РАН, ИЯФ СО РАН, НЦКЭМ СО РАН, ООО Тион
Нанесенные титан-магниевые катализаторы: новые данные о формировании и структуре активных центров и свойствах в полимеризации олефинов
Найдено, что нанесенные катализаторы состава TiCl4/MgCl2 (ТМК) с очень низким содержанием Ti (≤ 0,1 % масс.) обладают сверхвысокой активностью, более однородным составом соединений титана и являются удобной каталитической системой для исследования процессов формирования и структуры АЦ реальных нанесенных ТМК.
Методом препаративного фракционирования полипропилена (p-TREF) получены данные о влиянии содержания титана, предварительной обработки катализатора активатором (AlEt3) и введения внешнего донора на распределение АЦ по стереорегулярности при полимеризации пропилена. На основании этих данных предложены возможные структуры нестереоспецифических АЦ (алкильных соединений Ti(III), локализованных на грани (110) MgCl2) и схема их трансформации в АЦ с повышенной стереоспецифичностью.
д.х.н. Т.Б. Микенас, Е.И. Кошевой, М.И. Николаева, д.х.н. В.А. Захаров,
к.х.н. Л.Г. Ечевская, к.х.н. М.А. Мацько, к.ф.-м.н. А.А.
Шубин, к.х.н. А.А. Барабанов
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
“Скрытая” радикальность комплексов феррил-иона FeO2+ как главный фактор активности в разрыве С-Н связей
Aктивация C-H связей FeIII-гидроксидами на группе [FeO]2+ в основном феррильном состоянии FeIV=O определяется “скрытым” возбуждённым оксильным состоянием FeIII-O•, которое может обеспечить сверхактивность такого центра, присущую свободным радикалам.
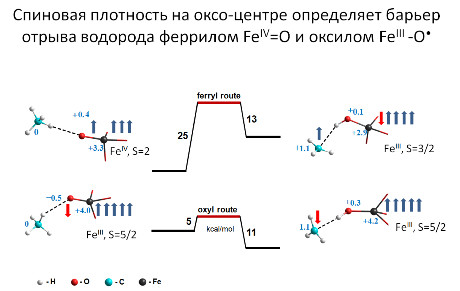
д.х.н. И.Л. Зильберберг, к.ф.-м.н. А.А. Шубин, к.ф.-м.н. С.Ф. Рузанкин,
В.Ю. Ковальский, Д.А. Овчинников, д.ф.-м.н., проф. Г.М. Жидомиров
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Получение мягких кислот Льюиса на основе фторированных органических соединений бора и исследование их каталитических свойств
Разработаны удобные способы получения органофторборанов, являющихся мягкими кислотами Льюиса, перспективными для использования в селективных гомогенных каталитических процессах. Органофторбораны могут быть получены в виде раствора в гексане путем взаимодействия стабильных и относительно легко получаемых калиевых солей органофторборатов с ионными жидкостями типа BMIM+Cl–·nAlCl3. На примере реакций алкилирования ароматических соединений показано, что получаемые органофторбораны являются мягкими кислотами Льюиса, селективно катализирующими алкилирование активированных субстратов. Установлено, что каталитические свойства органофторборанов определяются природой и количеством заместителей в окружении атома бора.
д.х.н. Н.Ю. Адонин, к.ф.-м.н. М.М. Шмаков, к.х.н. С.А. Приходько
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Исследования в области катализа, проведенные в Институте углехимии и химического материаловедения ФИЦ угля и углехимии СО РАН
Показана возможность целенаправленного регулирования физико-химических свойств катализаторов MnМW/SiO2 и MnМW-La/SiO2 (M = Na, K, Rb) и, соответственно, их активности в окислительной конденсации метана с образованием этана и этилена путем варьирования метода синтеза, типа щелочного металла и температуры прокаливания. Определен состав оптимального катализатора 2 мас. % Mn 0.8% Na 3 мас. % W/SiО2.
Разработаны и испытаны капиллярные микрореакторы непрерывного действия с новыми каталитическими покрытиями в селективном гидрировании 2-метил-3-бутин-2-ола. Исследована кинетика реакции на покрытиях Pd/(Ti,Ce)O2, Pd80Zn20/(Ti,Ce)O2 и Pd/TiO2.
Разработана методика синтеза препаратов аминозамещенного силанола в виде растворенных аморфных частиц около 1 нм, устойчивых к агрегации, адаптированных для иммобилизации фрагментов ДНК. Разработанные частицы являются новым типом систем доставки терапевтических ДНК в клетки и впервые будут использованы для создания нанокомпозитов, способных селективно воздействовать на внутриклеточный генетический материал.
Приготовлены Cu-катализаторы, нанесенные на диоксид титана, в виде активированной механической смеси, содержащей наноразмерный рутил и анатаз. Катализаторы исследованы методами РФА, БЭТ, ЭСДО и ЭПР. Показано, что наличие фазы рутила улучшает каталитические свойства Cu/TiO2 в реакции DeNOx при низких температурах.
Проведены промышленные испытания установки одностадийной каталитической очистки попутного нефтяного газа от сероводорода. Эффективность очистки более 97%. АО «СМП-НЕФТЕГАЗ», Республика Татарстан.
Введена в эксплуатацию промышленная установка каталитического окисления сероводорода в элементарную серу. АО «Конденсат», Республика Казахстан.
Разработаны каталитические блоки сотовой структуры, для очистки дымовых газовов от оксидов азота и серы, введена в эксплуатацию полупромышленная установка по каталитической очистке дымовых газов тепловых электростанций угольной генерации.
Разработан метод гидрогенизации барзаских углей при температуре 350-400°C на наноразмерном никельмолибденовом катализаторе с целью получения жидких продуктов в автоматизированной автоклавной установке при 50-55 атм. Получены предварительные данные о механизме превращения органической массы углей.
чл.-корр. РАН З.Р. Исмагилов
Институт углехимии и химического материаловедения ФИЦ УУХ СО РАН, г. Кемерово
Разработка и усовершенствование промышленных катализаторов и технологий
Создание технологии производства импортозамещающих катализаторов глубокой гидропереработки вакуумного газойля
Разработаны новые катализаторы, позволяющие обеспечить глубокую переработку нефтяного сырья: CoNiMo(ИК) для процессов гидроочистки вакуумного газойля (ВГО) и алюмосиликатный катализатор гидрокрекинга ВГО. Катализатор глубокой гидроочистки ВГО дает возможность получать сырье для каталитического крекинга, содержащее 200-500 м.д. серы при температуре гидроочистки на 5-20°C ниже и с выходами целевых продуктов на 4-13% больше, чем на современных импортных и отечественных катализаторах. Алюмосиликатный катализатор гидрокрекинга ВГО позволяет по сравнению с известными импортными катализаторами увеличить выход дизельной фракции на 4-5%, снизить уровень газообразования ниже 3%, уменьшить себестоимость в полтора раза за счет меньшего содержания Ni и Mo(W) в катализаторе.
академик В.Н. Пармон, д.т.н. А.С. Носков, к.х.н. О.В. Климов
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Упрощенная технология получения и переработки реакторного порошка сверхвысокомолекулярного полиэтилена
Разработана новая упрощенная технология получения и переработки реакторного порошка сверхвысокомолекулярного полиэтилена (РП СВМПЭ) в сверхпрочные и сверхмодульные изделия (пленки и пленочные волокна) методом твердофазной переработки. Рассмотрение технологии полимеризации и технологии переработки РП СВМПЭ в одном ключе позволило учесть формирование нужной морфологии и наноструктуры получаемых РП для стадии твердофазной переработки. Наработана укрупненная партия РП СВМПЭ, уточнены требования к размеру и плотности РП. Реализована стадия переработки с получением волокон с прочностью 3,3-3,6 ГПа и модулем 120-150 ГПа. Разработка защищена тремя патентами РФ. Разрабатываемая технология отличается оригинальностью и не имеет аналогов в производстве.
чл.-корр. РАН С.С. Иванчев
Санкт-Петербургский филиал Института катализа им. Г.К. Борескова СО РАН, г. Санкт-Петербург
Разработка российского аналога нового поколения перфторированных мембран
Для водород-кислородных топливных элементов, предназначенных для использования в наземной, подводной и воздушной технике, разработан российский аналог нового поколения перфторированных мембран типа Aquivion, работающих при более высоких температурных условиях, чем мембраны Nafion (фирма DuPont, США), и испытан в мембранно-электродных блоках в Крыловском государственном научном центре. Изучены особенности тонкой структуры мембран типа Aquivion и условия оптимизации их протонной проводимости.
чл.-корр. РАН С.С. Иванчев
Санкт-Петербургский филиал Института катализа им. Г.К. Борескова СО РАН, г. Санкт-Петербург
Каталитическая очистка отходящих газов от летучих органических соединений на катализаторах Pt/стекловолокно
Исследован процесс очистки отходящих газов от летучих органических соединений на платино-стекловолокнистых катализаторах.
Наработана партия катализатора 0.012%Pt/СВ с низким содержанием платины, которая успешно эксплуатируется на ПАО «Нижнекамскнефтехим» в дожиге летучих органических соединений в отходящих газах завода СКИ, обеспечивая уровень очистки не менее 99.99%.
к.х.н. Е.В. Ковалев, к.х.н. А.П. Сукнев, д.х.н. Б.С. Бальжинимаев
Институт катализа им. Г.К. Борескова СО РАН, г. Новосибирск
Экспериментальная оптимизация на пилотной установке нового процесса комплексной переработки биомассы березы в биотоплива и ценные химические продукты
В продолжение исследований по созданию фундаментальных основ процесса комплексной переработки биомассы березы (древесина, кора) в биотоплива и ценные химические продукты проведена экспериментальная оптимизация на пилотной установке стадий каталитического фракционирования древесины с получением ксилозы, микрокристаллической целлюлозы (МКЦ) и растворимого низкомолекулярного лигнина, каталитической деполимеризации МКЦ с получением глюкозных гидролизатов для синтеза биоэтанола, выделения биологически активного бетулина и суберинового связующего из бересты, получения твердых биотоплив с использованием связующих на основе лигнина и суберина, получения энтеросорбента из луба коры березы.
Наработаны опытные партии ксилозы, МКЦ, биоэтанола, бетулина, твердого биотоплива, энтеросорбента и проведены их исследовательские испытания. Разработан технологический регламент процесса комплексной переработки биомассы березы. Преимущества нового процесса по сравнению с аналогичными разработками: обеспечивается утилизация всей биомассы березы, включая лигнин и кору (тогда как в традиционных технологиях перерабатываются только полисахариды), что делает процесс безотходным; наряду с биотопливами нарабатываются продукты с высокой добавленной стоимостью из коры и лигнина, что снижает себестоимость производства биотиплив; в технологическом цикле отсутствуют токсичные, экологически опасные и коррозионно-активные реагента, что позволяет снизить, по сравнению с традиционными технологиями, затраты на утилизацию отходов и на мероприятия по охране труда и окружающей среды.
д.х.н. Б.Н. Кузнецов
Институт химии и химической технологии СО РАН, г. Красноярск
Разработка катализаторов гидроочистки. Проектирование катализаторной фабрики в г. Омске
По результатам НИР “Разработка энергоэффективной технологии селективной гидроочистки бензинов каталитического крекинга с сохранением октанового числа” был разработан катализатор CoMoS/CoSx/SiO2 и на его основе технология селективной гидроочистки бензина каталитического крекинга, реализуемая по схеме с предварительным гидрированием диеновых углеводородов и последующим его разделением на легкую и тяжелую фракции в ректификационной колонне. Тяжелая фракция далее подвергается селективной гидроочистке в двух параллельных реакторах. Для разработанной технологической схемы выбрано технологическое оборудование.
Осуществляется проектирование катализаторной фабрики в г. Омске (АО “АО Газпромнефть-ОНПЗ”), позволяющей получать отечественные современные катализаторы гидрокрекинга, гидроочистки, каталитического крекинга.
Проектирование и строительство опытно-промышленной установки гидроконверсии гудрона на наноразмерном катализаторе мощностью 50 тыс. т/год на АО “Танеко”.
Проектирование современной установки по получению нафтила на ООО “Газпромнефтехим Салават”.
д.т.н., проф. В.М. Капустин
ОАО «ВНИПИнефть», Москва
Катализаторы эпоксидирования пропилена
Получены молибденовые катализаторы эпоксидирования пропилена гидропероксидом этилбензола на основе пероксида водорода и порошкообразного молибдена с использованием гликолей, а также спиртового раствора пероксида циклогексанона (1,1'-диоксидицикло-гексилпероксида) и молибденового порошка, обладающие высокой стабильностью и селективностью. Опытно-промышленные испытания, проведенные на пилотной установке эпоксидирования цеха №1122 НТЦ ПАО «Нижнекамск-нефтехим», показали, что эти катализаторы обеспечивают режим мягкого протекания реакции эпоксидирования пропилена, без резкого повышения температуры и давления в начале процесса, как это характерно для заводского комплексного молибденового катализатора (КМК). Новые катализаторы обеспечивают получение сопоставимых с КМК технологических показателей эпоксидирования пропилена: конверсия ГПЭБ 97%, селективность по оксиду пропилена 82%. Применение данных катализаторов вместо КМК позволит высвободить гидропероксид этилбензола из стадии приготовления катализатора и получить дополнительное количество оксида пропилена.
д.х.н., проф. Х.Э. Харлампиди
Казанский национальный исследовательский технологический университет, г. Казань
Катализатор и процесс синтеза дивинила из этанола; прочностные свойства катализаторов
Отработана технология производства катализатора для синтеза дивинила из этанола в промышленных условиях. Приготовлена опытная партия катализатора. Смонтирована полузаводская демонстрационная установка синтеза дивинила из этилового спирта для отработки технологических параметров процесса.
Методами термогравиметрии, рентгеноструктурного анализа, физико-механических испытаний изучалось влияние различных факторов (используемое сырье, технологические параметры и др.) на механическую прочность пылевидных катализаторов на основе оксидов алюминия для процессов дегидрирования изобутана и пропана и гранулированных катализаторов на основе оксидов железа для процесса дегидрирования этилбензола. Изучены основные закономерности формирования прочностных свойств алюмооксидных и железооксидных катализаторов; предложены рецептура и технологии производства катализаторов дегидрирования этилбензола с улучшенной на 80-100% механической прочностью.
д.т.н., проф. Г.Р. Котельников
ОАО НИИ «Ярсинтез», г. Ярославль
Исследование воздействия метилдиэтаноламина (МДЭА) на активированные никелевые катализаторы метанирования
В процессе эксплуатации агрегатов по производству аммиака большой единичной мощности наблюдаются уносы абсорбентов диоксида углерода в реактор метанирования. Это может приводить к увеличению газодинамического сопротивления и снижению каталитической активности.
Комплексом физико-химических и физико-механических методов исследованы характеристики и изучена каталитическая активность Ni-Al катализатора НИАП-07-01 (НКМ-1), Ni-цементсодержащего катализатора НИАП-07-07 (НКМ-7), а также ряда импортных контактов, как в их исходном состоянии, так и активированных с последующим воздействием на них водного раствора МДЭА в протоке азотоводородной смеси (АВС). Показано, что исследуемые катализаторы после воздействия на них водного раствора МДЭА в протоке АВС, в отличие от водного раствора поташа, практически сохраняют высокие значения механической прочности, общей удельной поверхности, пористости и каталитической активности. Обнаружено, что в процессе воздействия при температуре 320°C на активированные катализаторы водного раствора МДЭА в протоке АВС происходит их пассивация, сопровождающаяся повышением температуры и снижением активности. Установлено, что поверхность катализаторов блокируется МДЭА. Регенерация активированных катализаторов метанирования, подвергшихся воздействию водного раствора МДЭА, должна осуществляться путем их сушки в протоке АВС с последующим довосстановлением.
д.х.н., проф. Е.З. Голосман, к.т.н. В.Н. Ефремов
ООО “НИАП-КАТАЛИЗАТОР”, г. Новомосковск
Использование цементсодержащих катализаторов для получения аминов
Изучено влияние способа приготовления Cu-Zn- и Cu-Mg-содержащих композиций, синтезированных с использованием алюминатов кальция (талюма), в частности, последовательности внесения компонентов, на эффективность их каталитического действия в реакции аминирования н-бутанола. Лучшие результаты достигнуты при формировании катализаторов из двойной сложной соли на основе смешанных гидроксокарбонатов Cu-Zn или Cu-Mg. Установлено, что Cu-Mg-содержащие образцы уступают в эффективности каталитического действия Cu-Zn-аналогам.
На лучшем из Cu-Zn-цементных образцов исследована возможность влияния внешней диффузии на процесс гидроаминирования н-бутанола аммиаком с преимущественным образованием дибутиламина.
Предложен новый способ синтеза бутиламинов, в особенности с существенным преобладанием дибутиламина, с использованием модифицированных промышленных никельалюмокальциевих катализаторов другого назначения, известных под марками НКМ 4А и НКМ-7, компоненты в которых находятся в оксидной или солевой форме, соответственно. В оптимальных условиях на указанных контактах достигается конверсия бутанола на уровне аналога при селективности по дибутиламину примерно в 1,4 раза выше в сравнении с последним.
На экспериментальных и промышленных образцах Cu- и Ni-содержащих катализаторов исследованы основные направления превращений 2-этоксиэтанола в парофазной реакции его гидроаминирования аммиаком. Установлено, что никелевые катализаторы на алюмокальциевой основе имеют заметное преимущество в производительности по 2-этоксиэтиламину перед контактами на других носителях.
к.х.н. В.В. Белов
ГВУЗ “Украинский
государственный химико-технологический университет”, г.
Днепропетровск
д.х.н., проф. Е.З. Голосман
ООО “НИАП-КАТАЛИЗАТОР”, г. Новомосковск
Катализаторы для процессов гидроочистки, каталитического крекинга и риформинга
Реализованы проекты по импортозамещению катализаторов для процессов гидроочистки средних дистиллятов, катализаторов каталитического крекинга, каталитического риформинга и изомеризации на действующих мощностях Компании КНТ Групп и на вновь построенных в последние годы предприятиях (производство катализаторов для этих процессов в ОАО “Газпромнефть-Омский НПЗ”). На мощностях Компании КНТ Групп начат выпуск катализаторов гидрокрекинга и идет поиск применения их вместо импортных катализаторов. На ПАО “Нижнекамскнефтехим” и химзаводе им. Л.Я. Карпова (г. Менделеевск) ведутся исследовательские работы по организации производства катализаторов дегидрирования нормального бутана.
д.х.н. Р.С. Яруллин
ОАО "Татнефтехиминвест холдинг" г. Казань
Разработка отечественных каталитических технологий для производства высококачественных и экологически чистых сортов автобензинов
Создание отечественной технологии каталитического риформинга с непрерывной регенерацией катализатора для производства высококачественных автобензинов. Завершена разработка промышленной технологии производства катализатора для установки риформинга с непрерывной регенерацией. Выпущена первая промышленная партия катализатора RC-12. Проекту присвоен статус национального (Протокол № АТ-646пр от 20.12.2016 г.).
Создание технологии изомеризации С7-фракции для обеспечения производства экологически чистых сортов автобензинов (Изомалк-4). Проведены исследования по разработке промышленной технологии производства катализатора и технологии изомеризации С7-фракции. Разработана промышленная технологическая линия для производства катализатора изомеризации С7-фракции СИ-4. На заседании рабочей группы Минэнерго России отмечена актуальность разработки технологии изомеризации С7-фракции для производства экологически чистых сортов автобензинов.
к.х.н. А.Н. Шакун
ОOO “НПП Нефтехим”, г. Краснодар
Способ получения полупродукта присадки “Детерсол” путем алкилирования салициловой кислоты α-олефинами С16-С18 в присутствии макропористого сульфокатионита
Разработан новый способ получения полупродукта присадки “Детерсол” путем алкилирования салициловой кислоты α-олефинами С16-С18 в присутствии макропористого сульфокатионита. Промышленный сульфокатионит подвергается предварительной осушке и загружается в реактор. Сырьевая смесь высокомолекулярных олефинов с салициловой кислотой в заданном соотношении в интервале 1:1,05 – 1:1,14 смешивается в аппарате с мешалкой, нагревается до необходимой температуры и подается в реактор насосом, производительность которого обеспечивает время пребывания в реакторе от 3,0 до 30,0 ч. Процесс идет с высоким выходом (около 90%) и практически 100% селективностью. После выхода из реактора реакционная масса остужается и осуществляется анализ ее состава. В дальнейшем проводится очистка реакционной массы от не вступившей в реакцию салициловой кислоты методом вакуумной ректификации.
Преимуществом предложенного способа является получение алкилсалициловой кислоты в одну стадию. В процессе алкилирования олефиновый компонент полностью вступает в реакцию, практически отсутствуют продукты алкилирования ароматического кольца салициловой кислоты двумя молекулами высокомолекулярного олефина. Процесс не требует для его протекания каких-либо растворителей, что позволяет упростить отгонку непрореагировавших компонентов и снизить параметры этого процесса. Чистота целевого продукта оказывается более 99% масс.
д.т.н. А.В. Тыщенко
ПАО “Средневолжский НИИ по нефтепереработке”, г. Новокуйбышевск
19-23 сентября 2016 г., Лондон, Великобритания
http://conf.ict.nsc.ru/CR_22/en
19 - 23 сентября 2016 года в Лондоне прошла XXII Международная конференция по химическим реакторам ХИМРЕАКТОР-22. Конференция была организована Институтом катализа им. Г.К. Борескова СО РАН (Россия) совместно с Университетским колледжем Лондона (Великобритания). Традиционно она проводилась под эгидой Европейской Федерации по химической технологии (EFCE).
Широко известная на данный момент конференция ХИМРЕАКТОР имеет давнюю историю. Первое расширенное совещание, посвященное тематике химических реакторов, было проведено в Новосибирском Академгородке в Институте катализа СО РАН в 60-х годах прошлого века. Его организатором и идейным вдохновителем стал крупнейший ученый в области катализа и математического моделирования химических процессов и реакторов, один из основателей Института катализа, профессор Михаил Гаврилович Слинько. Через несколько лет совещание переросло в конференцию, а спустя еще несколько лет она получила статус международной и стала проводиться раз в два года на базе крупных научных центров различных стран мира, чья деятельность связана с развитием инновационных технологий в химии. Конференция неизменно собирает ученых с мировым именем, а также представителей крупных промышленных компаний, создавая платформу для обмена новыми идеями и обсуждения последних достижений в области химической технологии.
Конференция ХИМРЕАКТОР-22 была проведена под руководством профессора Александра Степановича Носкова (Институт катализа СО РАН) и профессора Марка-Оливье Коппенса (Университетский колледж Лондона). Международный программный комитет возглавил д.т.н., ведущий научный сотрудник ИК СО РАН Андрей Николаевич Загоруйко.

В работе конференции приняли участие около 200 специалистов из 30 стран мира.
Научная программа включала 6 пленарных лекций, 9 ключевых лекций, 68 устных и 100
стендовых докладов.
Отличительной особенностью конференций ХИМРЕАКТОР является сочетание докладов глубокого фундаментального характера с работами, имеющими важное практическое значение. Тематика конференции включала следующие направления:
В 2006 году организаторами конференции ХИМРЕАКТОР была учреждена Почетная лекция памяти ее основателя – профессора М.Г. Слинько. С тех пор программа конференции, её пленарная сессия открывается именно этой лекцией. Почетный лектор выбирается голосованием Международного научного комитета форума, который состоит из 35 ученых мирового уровня и возглавляется Научным руководителем Института катализа СО РАН академиком Валентином Николаевичем Пармоном. На конференции ХИМРЕАКТОР-22 в Лондоне эта честь выпала выдающемуся ученому, профессору Жильберу Фрома (Университет Техаса, США).

В своей лекции “Современные подходы к кинетике превращения углеводородов и конверсионных процессов” профессор Фрома представил передовые фундаментальные основы кинетики промышленных процессов превращения углеводородов. Он предложил новый подход к осуществлению конверсии углеводородных фракций или их производства из H2 и CO/CO2, который заключается в разделении всего процесса на детализированную сеть фундаментальных элементарных шагов, включающих подробный анализ сырья. Скорости элементарных шагов выражаются в терминах концепции “одиночного события кинетики”. Уникальность такого подхода заключается в отсутствии чрезвычайно сложных схем реакции в существующих технологиях и снижении зависимости от качества сырья.
Вице-президент Академии наук Китайской народной республики профессор Джингхай Ли (Институт технологического проектирования Академии наук Китая, Пекин, Китай) в своей пленарной лекции “Понимание виртуальной реальности химических реакторов: путь к мезомасштабным моделям” изложил свое представление о переходе к мезомасштабной стратегии в технологиях конструирования химических реакторов.
 Большое внимание привлекла лекция профессора Давида Уэста (Технологический центр SABIC, Шугар
Ленд, Техас, США) “Бифуркации в процессе окислительной димеризации метана”, посвященная влиянию нестационарных явлений на процесс окислительной димеризации
метана.
Большое внимание привлекла лекция профессора Давида Уэста (Технологический центр SABIC, Шугар
Ленд, Техас, США) “Бифуркации в процессе окислительной димеризации метана”, посвященная влиянию нестационарных явлений на процесс окислительной димеризации
метана.
Профессор Линн Гладден (Университет Кембриджа, Великобритания) посвятила лекцию “Магнитно-резонансные исследования гидродинамики жидкостных потоков в многофазных реакторах” применению магнитно-резонансных исследований к процессам, происходящим в химических реакторах. Магнитно-резонансные методы в настоящее время хорошо себя зарекомендовали в применении к визуализации структуры потоков газа, жидкости и распределения твердых фаз внутри химических реакторов. Они позволяют отслеживать химический состав и гидродинамику в пространстве и времени. Лекция была дополнена примерами реакторов различных типов, были продемонстрированы разные виды измерений, которые можно произвести с помощью магнитно-резонансных технологий.
Лекция профессора Мюррея Му-Янг (Университет Ватерлоо, Онтарио, Канада) “Системное проектирование биореакторов для производства биотоплива и биофармацевтики” была посвящена биоинженерным аспектам проектирования систем биореакторов с последующим их применением для осуществления биохимических процессов. Биореакторы используются в биотехнологической промышленности при производстве лекарственных и ветеринарных препаратов, вакцин, продуктов пищевой промышленности. При этом большое внимание уделяется процессу перемешивания, поскольку перемешивание с помощью механической мешалки или барботажное перемешивание приводит к гибели микроорганизмов и нежелательному пенообразованию. Профессор Му-Янг рассказал о новшествах в разработке специальных типов мешалок для биореакторов, где перемешивание осуществляется с помощью эрлифта и волновых устройств. Эти новшества могут существенно влиять на производительность биореактора. Большим дос-тижением являются газо-вихревые биореакторы, которые отличаются от традиционных аппаратов тем, что перемешивание в них осуществляется воздушным вихрем (без “мешалки” в жидкости), за счет перепада давления над поверхностью жидкости и трения воздушного потока об ее поверхность. Заключительная часть лекции была посвящена экологическим проблемам. Профессор Му-Янг считает, что следует изменить вектор геополитических обсуждений, направив международные усилия на защиту окружающей среды, развитие возобновляемых источников энергии и производство биотоплива.
В лекции профессора Сотирис И. Пратсинис (Швейцарская высшая техническая школа Цюриха, Цюрих, Швейцария) “Проектирование аэрозольных реакторов: суб-нано кластеры Pd на TiO2 для солнечно-светового удаления NO” были представлены преимущества аэрозольных реакторов по сравнению с технологией жидкостной химической обработки для синтеза материалов. Аэрозольные процессы, по мнению лектора, имеют явные преимущества для крупномасштабного синтеза материалов, они способны облегчить обработку частиц и формирование высокочистых материалов с уникальной морфологией.
Ключевые лекции также вызвали большой интерес и активные дискуссии. Их представили:
Профессор Майкл Стаматакис (Университетский колледж Лондона, Великобритания), “Точные и эффективные вычислительные платформы для кинетических реакций: на пути к принципиальным основам дизайна реакторов”;
Профессор Фрерих Кейл (Гамбургский технологический университет, Германия), “Мультимасштабное моделирование реакции и диффузии под влиянием анализа и дизайна химического реактора”;
Профессор Рууд ван Оммен (Технологический университет Делфта, Нидерланды), “Синтез наноструктурных материалов в реакторах с псевдоожиженным слоем”;
Профессор Йен Меткалф (Университет Ньюкасла, Великобритания), “Несмешанные реакции – их использование на реакторах периодического действия и мембранных реакторах”;
Профессор Майкл П. Гарольд (Университет Хьюстона, США), “Сокращение эмиссии Nox дизельными двигателями с помощью быстрой периодической пульсации”;
Профессор Менка Петковска (Факультет технологии и металлургии, Университет Белграда, Сербия), “Оценка потенциала вынужденных периодических операций химических реакторов – подход с использованием нелинейной частоты реакции”;
Профессор Энрико Тронкони (Технический университет Милана, Италия), “Последние достижения в области тепло-проводных микроструктурированных катализаторов”;
Профессор Андрей Загоруйко (Институт катализа им. Г.К. Борескова СО РАН, Новосибирск, Россия), “Новые структурированные каталитические системы – картриджи катализаторов на микроволокнистом носителе”;
Профессор Зинг-гай Жоу (Восточно-Китайский университет науки и технологии, Шанхай, Китай), “Изучение и контроль массообмена на металл-цеолитных катализаторах селективного окисления”.
Для участников конференции были организованы ежедневные экскурсии в лаборатории Департамента химического инжиниринга Университетского колледжа Лондона. Их проводили сотрудники Департамента.
 На закрытии конференции авторам выбранных представительной комиссией лучших стендовых
докладов были вручены дипломы и призы медиа-партнеров конференции – Королевского химического общества Великобритании
На закрытии конференции авторам выбранных представительной комиссией лучших стендовых
докладов были вручены дипломы и призы медиа-партнеров конференции – Королевского химического общества Великобритании
Материалы конференции будут опубликованы в специальных выпусках журналов издательства Эльзевир: Chemical Engineering & Processing: Process Intensification и Chemical Engineering Journal.

Организаторы уже получили приглашения от известных университетов мира на проведение следующего форума в 2018 году. Выбор места проведения традиционно будет сделан Международным научным советом конференции ХИМРЕАКТОР.
Материал подготовили
Т.В. Замулина, С.С. Логунова, А.Н. Загоруйко,
А.М. Ершова, А.С. Носков
(ИК СО РАН, Новосибирск)
Tool allows scientists to submit and search for safety information not publicly cataloged elsewhere
A nonprofit group today released a database tool chemists can use to share information about hazardous chemical reactions. Called the Chemical Safety Library, the tool was developed by a group that included representatives from pharmaceutical companies and academic institutions.
“We feel this will be a valuable and unique set of data that is currently not available and should advance safety for all researchers,” says Carmen Nitsche, executive director for business development in North America at the Pistoia Alliance, which brings together companies, vendors, publishers, and academic groups to address research and development challenges in the life sciences industry.
The project started when chemists at Bristol-Myers Squibb were looking for a better way to catalog and share information about lab accidents and other adverse events. Eventually the project landed at Pistoia.
“I didn’t know if we were going to get any interest” in putting together the library, says Mark Manfredi, a business capability manager at Bristol-Myers Squibb. “But right from the first meeting, we had several organizations that were interested in participating.”
To use the Chemical Safety Library, chemists must first register for an account. They can then start entering reaction information, including specific reagents as well as reaction class, hazard category, scale, warning message, and additional information such as a literature reference. Pistoia worked with Millipore Sigma and Biovia to preload more than 75,000 reagents to help ensure accuracy. Library administrators review submitted reaction entries to ensure they are appropriate.
Chemists may search the library for particular reactions or reagents or even download the full data set. An organization could incorporate downloaded data into an electronic laboratory notebook system to issue an alert when a particular combination of reagents associated with a known hazard is entered. Bristol-Myers Squibb is already using the data in an electronic laboratory notebook system and an ordering system, Manfredi says.
Pistoia sees the current library tool as an experiment to gather information about the willingness of the community to populate and use the database, Nitsche says. Pistoia will analyze database use to determine the resources and technology needed to sustain the library long-term.
The library will be a “wonderful resource” for researchers to use as an additional source of information when doing hazard and risk assessments of experiments, comments Bettyann Howson, chair of the American Chemical Society’s Committee on Chemical Safety. ACS also publishes C&EN. C&EN plans to encourage scientists who submit safety letters to also enter the information into the Chemical Safety Library.
Zeolites synthesized with transition state mimics exhibit enhanced activity and product selectivity
Endowed with a network of interconnected, molecule-sized pores and channels, zeolites have been used in chemical separations, oil refining, and catalysis for decades. Yet scientists still tend to use a trial-and-error approach to find the best member of this large family of crystalline materials for a given application.
Researchers in Spain may have come up with a way to shorten and reduce the cost of that time-consuming search process. Their strategy is to tailor-make zeolite catalysts with pores that closely match the proposed transition state structure of a given reaction. They find that the customized zeolite outperforms commercial zeolite catalysts commonly used for that reaction (Science 2017, DOI: 10.1126/science.aal0121).
In one example of this approach, the team, which was led by Avelino Corma, the director of the Institute of Chemical Technology at Polytechnic University of Valencia, produced catalysts for the production of xylene from toluene. That reaction, which is carried out commercially mainly with ZSM-5 and mordenite zeolites, converts two toluene molecules to one molecule of benzene and one of the dimethylbenzene isomers (o-, m-, or p-xylene).
Based on earlier studies, researchers have proposed that the reaction proceeds by way of a transition state featuring a complex made of two phenyl rings joined by a positively charged carbon atom. A zeolite catalyst would need pores big enough to accommodate that structure, which is larger than the reactants and the products.
So the Valencia team selected a diphenylphosphonium compound that closely mimics the structure and charge of the transition state and used it as a structure directing agent (SDA) to synthesize a zeolite. All zeolites are aluminosilicate minerals. The team produced their zeolite catalyst, called ITQ-27, by allowing the mineral to crystallize around the diphenylphosphonium molecules. They then heated the zeolite to remove the organic material, leaving an aluminosilicate frame with pores matching the SDA and the transition state structure.
Then the team compared the performance of ITQ-27 with that of ZSM-5, mordenite, and other zeolites. They found that although some of the zeolites had a larger number of catalytic sites than ITQ-27, the custom-made ITQ-27 was the most active catalyst. It was also as selective or more selective, meaning it produced fewer by-products, than the other catalysts.
In another example of the transition state strategy, Corma and coworkers successfully synthesized zeolites for ethylbenzene isomerization.
This approach, which represents “a new paradigm in zeolite science, may accelerate discovery of highly active and selective zeolite catalysts,” says Roberto Millini, a specialist in materials chemistry and catalysis at Eni, an oil and gas company based in Italy.
Millini notes that in addition to improving the efficiency of petrochemical processes, this strategy may enable new applications for crystalline porous materials. For example, these materials may provide environmentally benign routes for converting biomass to biofuels and chemicals.
Bifunctional metal agents reversibly coordinate heterocycles and derivatize them at remote sites
A bimetallic reagent’s anchoring palladium (near center) coordinates with the nitrogen atom of a substrate (quinoline in this case), positioning the reagent’s second palladium (lower right) to activate a remote C–H bond.
Chemists often use C–H activation to help replace specific hydrogen atoms in organic compounds with complex functional groups. This strategy generally involves covalently bonding a reagent containing a C–H-activating group, such as a palladium atom, to a substrate that already has a directing functional group. The directing group steers the C–H activator to the desired C–H bond. Once the bond breaks, a new functional group replaces hydrogen and the directing group is removed.
A new class of bimetallic reagents now uses enzyme-inspired reversible metal coordination, instead of covalent bonding, to achieve C–H activation and functionalization in nitrogen heterocycles, with no need to preinstall or later remove a directing group. The reagents, designed by Jin-Quan Yu and coworkers at Scripps Research Institute California, also activate remote C–H bonds that have been difficult to reach or completely inaccessible with other synthetic techniques (Nature 2017, DOI: 10.1038/nature21418).
C–H activation hasn’t worked well with heterocycles because metal activators tend to coordinate with heteroatoms, interfering with site selectivity. Yu and coworkers have now turned that problem into an advantage. In their new bimetallic reagents, one palladium atom is designed intentionally to coordinate reversibly with the heteroatom of a substrate, positioning the second metal to activate a remote C–H bond.
The reagents use distance and geometry constraints to focus on specific target sites on substrates, like enzymes do. They coordinate reversibly with substrate molecules, derivatize them, detach after activation, and then move on to activate other substrate molecules, also like enzymes do. The reagents work stoichiometrically or catalytically, and relatively large amounts are required. But their efficiency can potentially be improved, experts say.
Yu and coworkers used the new reagents to alkenylate a range of nitrogen heterocycles, including phenylpyridine, quinoline, and the anticancer natural product camptothecin, none of which could previously be functionalized in the same way using C–H activation, Yu says.
Motomu Kanai of the University of Tokyo comments that the technique is “very powerful,” noting that it could ease drug design by making it possible to modify compounds at positions other methods cannot easily reach. Victor Snieckus of Queen’s University in Ontario calls the discovery “a major leap forward in synthetic aromatic substitution chemistry.” And Kian Tan, who leads the Chemical Technology-Synthesis group at Novartis in Cambridge, Mass., says the templates are amazingly well designed and versatile, making it “easy to envision creating libraries of templates to access different selectivity patterns.”
Separation method corrals key compounds to improve petrochemical processing and pollution assessment
 Crude oil is an unruly soup of tens of thousands of different organic compounds, and this diversity makes it difficult to pick out individual molecules from the crowd for analysis using standard tools like mass spectrometry. Despite the vast quantities of crude oil used globally each day, much remains unknown about its chemical composition, which can vary dramatically from one oil field to the next.
Crude oil is an unruly soup of tens of thousands of different organic compounds, and this diversity makes it difficult to pick out individual molecules from the crowd for analysis using standard tools like mass spectrometry. Despite the vast quantities of crude oil used globally each day, much remains unknown about its chemical composition, which can vary dramatically from one oil field to the next.
So a method that separates crude oil into a dozen fractions, based on their chemical properties, now promises to help measure levels of molecules that could trigger corrosion in a pipeline, or pinpoint the most toxic compounds in an oil spill (Anal. Chem. 2017, DOI: 10.1021/acs.analchem.6b04202).
Fractionation is not a new approach to simplifying oil analysis. One of the most common methods, dubbed SARA, uses chromatography to split the oil into four broad classes: saturates, aromatics, resins, and asphaltenes. But this separation is largely based on the molecules’ solubilities in the solvent being used, and many chemical classes remain obscured within the mélange in each fraction.
In contrast, the new method developed by Steven J. Rowland of the University of Plymouth and coworkers is particularly good at teasing apart polar compounds containing nitrogen, sulfur, or oxygen—often responsible for poisoning oil-processing catalysts—which conventional analytical methods struggle to identify.
The procedure is not based on radical innovation: It relies on a series of columns filled with commercial ion exchange resins and silica, making the method reproducible, relatively simple, and inexpensive. “The real novelty is putting it all together,” says Ryan P. Rodgers, director of the Future Fuels Institute at Florida State University, who was not involved with the work. By deploying the separation columns in the right order and eluting the crude with a series of increasingly polar solvents, the method isolates molecules depending on how well their functional groups stick to each type of column. This yields fractions that are each dominated by a particular chemical class: sulfoxides, quinolines, carbazoles, fluorenones, and more.
After analyzing each fraction with techniques such as gas chromatography-mass spectrometry (GC-MS), the team identified dozens of specific compounds. Some of them, such as thioxanthones, were previously unknown in crude oil. The method achieves “a better separation between different classes of chemicals,” says Sonnich Meier of the Institute of Marine Research. “It’s the best I’ve seen.”
Meier has been working with Rowland’s team for the past three years, and plans to use the technique to single out the compounds in crude oil that are toxic to fish embryos. Polyaromatic hydrocarbons account for about 60% of oil’s toxicity, says Meier, but the culprits responsible for the remainder of the effect are unknown. “There are thousands of compounds in oil that we just ignore, because we’re not good at analyzing them,” he says. These data will feed into a new risk assessment on the potential exploitation of oil reserves in the Lofoten region of northern Norway, currently a matter of fierce debate in the country.
Meanwhile, the oil industry increasingly wants to know the precise composition of a crude oil before investing billions in extracting and refining it. The world’s declining production of low-sulfur oil, known as sweet crude, means there is more reliance on crudes that contain higher proportions of heteroatoms and heavier compounds, requiring more refining and presenting new processing challenges, such as pipeline corrosion or blockages. The oil industry has expressed interest in the method, Rowland says.
Potential applications include improved heat-transfer fluids and new inorganic thermoelectric materials
A new study proposes that chemical bonding of surface atoms (orange) of a nanocrystal to solvent ions (blue) of a molten inorganic salt is required for colloidal stability. Outer spheres are solvent ions.
A colloidal solution is a uniform dispersion of two completely different types of components—particles or droplets of one phase, called the solute, in a second, typically liquid, phase, called the solvent. Milk, a colloid of tiny globs of butterfat in water, is one of the most familiar.
Up to now, colloids have nearly exclusively been formed in polar solvents such as water or in organic solvents. Dispersions of silica particles in molten inorganic salts have been made before, but they have been metastable—that is, their colloidal form eventually breaks down.
Now, Dmitri V. Talapin of the University of Chicago and coworkers report the first stable colloids of nanoparticles in several types of molten inorganic salts (Nature 2017, DOI:10.1038/nature21041). They also propose a mechanism behind the new colloids’ stability.
Such nanoparticle colloids could improve the heat transport properties of molten salts, which are used as heat-transfer fluids in nuclear reactors and solar-energy facilities. The work could lead to new types of nanomaterials by making it possible to synthesize them in molten salts at temperatures much higher than are currently feasible with ordinary solvents. And the new colloids could result in more-efficient inorganic thermoelectric materials by enabling researchers to engineer them in new ways.
Over the past few years, Talapin and coworkers developed an improved understanding of the inorganic surface chemistry of different materials that enabled them to select combinations of materials that form stabilized colloids. They make stable colloid dispersions by transferring surface-modified nanocrystals from organic solvents to molten salts or by mixing modified-nanocrystal powders directly with molten salts. In this study, they incorporated metal, semiconductor, rare-earth, and magnetic nanocrystals into AlCl3/NaCl/KCl and six other types of molten inorganic salts.
Conventional colloids are stabilized by electrostatic or steric forces that prevent solute particles from aggregating. But Talapin’s group proposes a new mechanism for the stability of molten inorganic-salt colloids: Strong solute-solvent chemical bonds that form at the nanocrystal surface induce partial ordering of the molten salt around each nanocrystal. The researchers’ theoretical analyses and molecular dynamics modeling suggest that this induced ordering in the molten salt prevents the particles from combining.
Colloids expert Håkan Wennerström of Lund University says he remains skeptical for now about some technical details of the mechanistic proposal. However, he says, the study’s observations are “clear-cut and carefully made” and the chemical bonding-based mechanistic proposal is “convincing.” It’s important “for materials science and technical applications to have methods for handling metals, alloys, and semiconductor materials in the form of colloidal particles in a solvent that can take a high temperature like a molten salt,” Wennerström adds.
Chemical & Engineering News
|
2nd Green and Sustainable Chemistry Conference (2GSCC 2017) Berlin, Germany |
|
|
III Всероссийская молодежная конференция “Достижения молодых ученых: химические науки” Уфа, Республика Башкортостан, Россия |
|
|
3-я Всероссийская молодежная научная конференция с международным участием “Экологобезопасные и ресурсосберегающие технологии и материалы” Улан-Удэ, Республика Бурятия, Россия |
ertm3@mail.ru |
|
29th European Symposium on Applied Thermodynamics (ESAT 2017) Bucharest, Romania |
|
|
IV Школа-конференция молодых ученых “Неорганические соединения и функциональные материалы” Новосибирск, Россия |
icfm.2017@gmail.com |
|
III Российский конгресс по катализу “Роскатализ-2017” Нижний Новгород, Россия |
|
|
Международная научно- практическая конференция “Нефтегазопереработка-2017” Уфа, Республика Башкортостан, Россия |
|
|
Российский нефтегазохимический форум и XXV Международная выставка “Газ. Нефть. Технологии” Уфа, Республика Башкортостан, Россия |
|
|
7th Slovenian-Serbian-Croatian Symposium on Zeolites Ljubljana, Slovenia |
|
|
VI Международная научно- техническая конференция “Альтернативные источники сырья и топлива” (АИСТ-2017) Минск, Беларусь |
|
|
25th North American Meeting (NAM) of the Catalysis Society Denver, Colorado, USA |
|
|
III Всероссийская молодежная научно-техническая конференция “Инновации в материаловедении” (ИнМат 2017) Москва, Россия |
|
|
Всероссийская научная конференция с международным участием “Современные проблемы органической химии” Новосибирск, Россия |
|
|
IX Международная конференция “Полимерные материалы пониженной горючести” Алматы, Республика Казахстан |
|
|
8th International IUPAC Symposium “Macro- and Supramolecular Architectures and Materials: Multifunctional Materials and Structures” (MAM-17) Sochi, Russia |
|
|
European Biomass Conference & Exhibition (EUBCE 2017) Stockholm, Sweden |
|
|
IV Всероссийская научно-практическая молодежная конференция “Новые материалы, химические технологии и реагенты для промышленности, медицины и сельского хозяйства на основе нефтехимического и возобновляемого сырья” Уфа, Республика Башкортостан, Россия |
|
|
VII Symposium on Hydrogen, Fuel Cells and Advanced Batteries (HYCELTEC 2017) Porto, Portugal |
|
|
9-th International Symposium “Molecular Mobility and Order in Polymer Systems” Saint Petersburg, Russia |
|
|
19th IUPAC International Symposium on Organometallic Chemistry Directed Towards Organic Synthesis (OMCOS 2017) Jeju Island, Korea |
|
|
7th International Congress on Ionic Liquids (COIL 7) Mont Tremblant, Quebec, Canada |
http://www.engconf.org/conferences/chemical-engineering/conference-on-ionic-liquids-coil |
|
IX Voevodsky Conference “Physics and Chemistry of Elementary Chemical Processes” (VVV-100) Novosibirsk, Russia |
|
|
Meeting of the Spanish Catalysis Society (SECAT'17) Oviedo, Spain |
|
|
XXI International Conference on Chemical Thermodynamics in Russia (RCCT-2017) Novosibirsk, Russia |
|
|
II Международная научно- техническая конференция “Новые материалы и технологии глубокой переработки сырья” Москва, Россия |
|
|
Heterogeneous Catalytic Hydrogenation (a training course) Lisbon, Portugal |
http://www.rsc.org/events/detail/23259/heterogeneous-catalytic-hydrogenation |
|
II Всероссийская научная конференция с международным участием “Актуальные проблемы адсорбции и катализа” Плес, Россия |
|
|
Международная научно- техническая конференция “Нефтехимический синтез и катализ в сложных конденсированных системах” Баку, Азербайджан |
|
|
VI Всероссийская школа- конференция молодых ученых “Органические и гибридные наноматериалы” Иваново, Россия |
|
|
20th European Symposium on Organic Chemistry (ESOC 2017) Cologne, Germany |
|
|
2nd International Conference on New Photocatalytic Materials for Environment, Energy and Sustainability (NPM-2) Ljubljana, Slovenia |
|
|
22nd International Symposium on Olefin Metathesis and Related Chemistry (ISOM 2017) Zürich, Switzerland |
|
|
22nd EuCheMs Conference on Organometallic Chemistry (EuCheMs OMC 2017) Amsterdam, the Netherlands |
|
|
46th IUPAC World Chemistry Congress (IUPAC-2017) Sao Paulo, Brazil |
|
|
9th International Seminar on Flame Structure (9ISFS) Novosibirsk, Russia |
|
|
Liverpool Catalysis Summer School Liverpool, Great Britain |
http://www.liv.ac.uk/chemistry/events/catalysis-summer-school-2017 |
|
15th International Conference on Carbon Dioxide Utilization (ICCDU XV) Shnaghai, China |
|
|
18th International Symposium on Relations between Homogeneous and Heterogeneous Catalysis (ISHHC XVIII 2017) Sydney, Australia |
|
|
8th International Conference on Green and Sustainable Chemistry (GSC-8) Melbourne, Australia |
|
|
2-я Российская конференция “Графен: молекула и 2D кристалл” Новосибирск, Россия |
|
|
Молодежная конференция “Применение зондовой микроскопии в научных исследованиях” Екатеринбург, Россия |
|
|
13th European Congress on Catalysis (Europacat 2017) Florence, Italy |
|
|
11th Triennial Congress of the World Association of Theoretical and Computational Chemists (WATOC 2017) Munich, Germany |
|
|
Международная конференция по органической химии “Байкальские чтения-2017” и Научно-практический круглый стол по вопросам развития малотоннажной химии Иркутск, Россия |
|
|
Международная конференция “Сканирующая зондовая микроскопия - 2017” (Scanning Probe Microscopy 2017) Екатеринбург, Россия |
|
|
4th Central and Eastern European Conference on Thermal Analysis & Calorimetry (CEEC-TAC4) Chisinau, Moldova |
|
|
XIX Euroanalysis Conference Stockholm, Sweden |
|
|
15th International Conference on Environmental Science and Technology (CEST 2017) Rhodes, Greece |
|
|
8th World Congress on Oxidation Catalysis “Oxidation processes: challenges and solutions” (WCOC 2017) Krakow, Poland |
|
|
XII European Workshop Meeting on Innovation in Selective Oxidation (ISO’17) Krakow, Poland |
|
|
IV International Conference “Catalysis for Renewable Sources: Fuel, Energy, Chemicals” (CRS-4) Rimini, Italy |
|
|
XXIX Симпозиум “Современная химическая физика” Туапсе, Россия |
|
|
Международная конференция “Возобновляемые растительные ресурсы: химия, технология, медицина” (Renewable Resources RR 2017) Санкт-Петербург, Россия |
|
|
II симпозиум “Современные проблемы нанокатализа” с участием зарубежных ученых (NANOCAT-2017) Киев, Украина |
|
|
7th IUPAC Conference on Green Chemistry (7th ICGC) Moscow, Russia |
|
|
IX Научно-практическая конференция “Сверхкритические флюиды: фундаментальные основы, технологии, инновации” Сочи, Россия |
|
|
Международный Российско- Казахстанский Симпозиум “Углехимия и экология Кузбасса” Кемерово, Россия |
|
|
Global Conference on Catalysis and Reaction Engineering (GCR-2017) Las Vegas, USA |
|
|
V Всероссийская научная конференция “Теоретические и экспериментальные исследования процессов синтеза, модификации и переработки полимеров” Уфа, Республика Башкортостан, Россия |
|
|
Всероссийская конференция по квантовой и математической химии Уфа, Республика Башкортостан, Россия |
|
|
II Международная научно- практическая конференция “Графен и родственные структуры: синтез, производство и применение” Тамбов, Россия |


