
Поздравление
В.Б.Казанский (к 75-летию со дня рождения)
М.Г.Слинько
"Истоки и основы журнала "Катализ в промышленности"
Е.З.Голосман
"40 лет ИКАРУ "НИАП"
Е.З.Голосман
"Научный потенциал Тульского региона"
Т.В.Замулина, А.Н.Загоруйко, В.Я.Якововлев
"Химреактор-17"
За рубежом
Приглашения на конференции
(К 75-ЛЕТИЮ СО ДНЯ РОЖДЕНИЯ)
28 июня 2006 года действительному члену Российской академии наук, главному редактору журнала "Кинетика и катализ", заведующему лабораторией радиоспектроскопических и оптических методов изучения механизма гетерогенного катализа Института органической химии им. Н.Д. Зелинского РАН Владимиру Борисовичу Казанскому исполнилось 75 лет.

Владимир Борисович Казанский - крупный ученый в области катализа, спектроскопии, квантовой химии, химии и физики поверхности. Основным направлением работ В.Б. Казанского является изучение механизма гомогенного и гетерогенного катализа с использованием современных спектроскопических методов и квантово-химических расчетов. Эти работы были начаты еще в Институте химической физики АН СССР в конце пятидесятых годов. В то время арсенал спектроскопических методов был крайне ограничен и в основном сводился к использованию в катализе ИК спектроскопии. В связи с развитием метода электронного парамагнитного резонанса (ЭПР), первые работы В.Б.Казанского и его сотрудников были направлены на внедрение этого метода в катализ. В то время они не имели аналогов в нашей стране и заметно опережали подобные исследования, проводившиеся за рубежом. Полученные результаты продемонстрировали большие возможности метода ЭПР для изучения массивных и нанесенных оксидных катализаторов, а также свойств адсорбированных свободных радикалов. В частности, методом ЭПР впервые были зарегистрированы поверхностные координационно-ненасыщенные ионы переходных металлов, параметры спектров которых чувствительны к адсорбции молекул. На оксидах и в нанесенных системах впервые были обнаружены радикальные формы адсорбированного кислорода О2- и О-.
После перевода в 1967 г. лаборатории В.Б. Казанского в Институт органической химии им. Н.Д. Зелинского, арсенал используемых в его работах спектроскопических методов исследований значительно расширился. Кроме ЭПР, в него вошли люминесценция, спектроскопия диффузного рассеяния света в видимой и ультрафиолетовой области, ЯМР. Особенно плодотворным оказалось использование метода ИК спектроскопии диффузного отражения в широком спектральном диапазоне. В сотрудничестве с профессором Г.М. Жидомировым были начаты квантово-химические расчеты механизмов различных каталитических реакций. Они широко использовались и для более углубленной интерпретации спектроскопических данных. Была детально исследована природа Бренстедовских и Льюисовских кислых центров в цеолитах и механизм каталитических превращений углеводородов на гетерогенных и гомогенных кислотных катализаторах. Эти исследования способствовали широкому распространению спектроскопических методов и квантово-химических расчетов в каталитических исследованиях и значительно расширили и углубили представления о структуре поверхности оксидных катализаторов, природе активных центров и механизме кислотного и кислотно-основного гомогенного катализа.
В выполненных в 80-х годах работах В.Б.Казанского были существенно развиты представления о природе поверхностных кислых гидроксильных групп. Сделанный в этих работах вывод о ковалентной природе этих групп является сейчас общепринятым. На основании спектроскопических данных и квантово-химических расчетов была предложена рациональная систематика кислых гидроксильных групп, объясняющая зависимость их силы от содержания в кристаллической решетке цеолитов алюминия. Было дано новое толкование природы адсорбированных алкилкарбениевых ионов.
Одно из современных направлений работ В.Б. Казанского связано с изучением локализации в высококремниевых цеолитах двухвалентных катионов, которые придают им необычные каталитические свойства в реакциях дегидрирования парафинов, селективного восстановления углеводородами оксидов азота и селективного окисления углеводородов. В этих исследованиях было доказано образование пространственно разделенных кислотно-основных пар в которых положительный заряд двухвалентных катионов компенсируется лишь частично в результате чего они приобретают свойства Льюисовских сверхкислот.
Всеобщее признание получил цикл работ В.Б. Казанского по изучению природы жидких сверхкислот и механизма кислотного катализа с их участием. В этих работах впервые дано объяснение парадоксу, согласно которому безводные сверхкислоты диссоциированы лишь в очень малой степени, в то время как более высокая степень их диссоциации в водных растворах одновременно сопровождается уменьшением их силы. Объясняется это более низкими энергиями сольватации молекулами сверхкислот протонов, что делает их диссоциацию по сравнению с водными растворами менее благоприятной. С другой стороны, более низкая энергия сольватации придает протонам безводных сверхкислот более высокую химическую активность, что с избытком компенсирует их меньшую концентрацию. Развитие этих представлений на примере реакции алкилирования изо-парафинов олефинами в безводной серной кислоте позволило предложить новый механизм этой реакции.
В последние годы В.Б. Казанским предложен новый спектроскопический критерий оценки степени активации адсорбированных молекул в результате их поляризации активными центрами кислотно-основных катализаторов. В дополнение к низкочастотным сдвигам ИК-полос валентных колебаний в качестве индекса реакционной способности предлагается использовать интенсивности полос поглощения, которые напрямую связаны с поляризацией соответствующих химических связей. Плодотворность этого подхода уже подтверждена наблюдением более высокой интенсивности колебательных полос поглощения от более кислых гидроксильных групп и необычных ИК спектров адсорбированных молекул углеводородов
В лаборатории В.Б. Казанского разработана методика проведения каталитических превращений углеводородов в сверхкритических условиях в отсутствие растворителей. Сравнительные исследования реакций изомеризации н-бутана в изобутан, алкилирования изобутана бутиленом и олигомеризации бутиленов продемонстрировали преимущества сверхкритических условий по сравнению с традиционными газофазными и жидкофазными реакциями. Проведение реакции в сверххкритических условиях значительно повышает производительность катализаторов и увеличивает время их жизни по сравнению с длительностью работы для аналогичных реакций в газовой фазе.
В.Б. Казанский - автор более 650 научных работ, обзоров и патентов, около 200 из них опубликованы в международных журналах. Полученные результаты докладывались практически на всех важнейших международных и национальных конференциях по катализу. На многих из них В.Б. Казанский выступал в качестве пленарного докладчика. Его работы нашли широкое международное признание. Свидетельством этого является, в частности, то, что в течение ряда лет он входил в состав редколлегий международных журналов "Journal of Сatalysis", "Applied Catalysis", "Microporous and Mesoporous Materials", "Advances in Catalysis", а в настоящее время включен в состав редколлегии журнала "Сatalysis Letters".
Научную работу В.Б. Казанский успешно сочетает с преподавательской деятельностью. В течение ряда лет он читал лекции по физической химии и катализу в Московском физико-техническом институте, а в настоящее время является заведующим кафедрой физической химии и читает лекции по катализу в Высшем химическом колледже РАН. Под его руководством защищены свыше 25 кандидатских диссертаций, а 8 его учеников стали докторами наук.
Владимир Борисович находится в расцвете творческих сил и энергично воплощает свои замыслы в жизнь. Научный совет по катализу и редакция "Каталитического бюллетеня" сердечно поздравляют Владимира Борисовича с юбилеем, желают ему здоровья и дальнейших творческих успехов.
(Выступление на конференции, посвященной 5-летию
научно-технического журнала "Катализ в промышленности" 28 февраля 2006 г.)
Вспомним время и обстановку, когда возникла необходимость создания нового журнала по катализу.
Шоковая терапия и реформы 90-х годов привели к резкому снижению научно-технического потенциала химических отраслей страны. Развал Советского Союза был делом искусственным и его спровоцировали руководящие "товарищи"-предатели. За годы смуты и бездарных реформ мы потеряли огромную собственность, нажитую коллективным трудом химиков. Потери более чем в два раза превысили ущерб, причиненный в годы Великой Отечественной войны.
Распад Советского Союза явился геополитической катастрофой века. Отделение бывших республик СССР нанесло ущерб как нашей химической промышленности, так и промышленности самих республик. Получили развитие негативные процессы: были разрушены ранее установленные связи между заводами, разгромлены все отраслевые организации и институты, в том числе и журнал "Химическая промышленность" - старейший печатный орган химических отраслей. Отраслевые институты оказались без молодых кадров, без заказов, без перспектив.
В Академии наук длительное время считалось, а во многих институтах считается и сейчас, что химическая технология – это наука второго сорта. Именно поэтому академические журналы не печатали статьи по промышленному катализу. Только журнал "Теоретические основы химической технологии" является исключением, но объем возможных статей в нем ограничен.
В этой обстановке остро ощущалась необходимость нового журнала в области промышленного катализа. Решая вопрос об организации журнала и определении его структуры и содержания, мы обратились к нашей истории становления химической промышленности. История является идеальным полигоном для определения путей развития отрасли. Обращаясь к истории и изучая её, мы устанавливаем непрерывность творчества.
Становление катализа, химической технологии и химической промышленности произошло в конце 20-х годов. Руководители Советской России очень быстро осознали жизненную необходимость создания полноценной собственной научно-технической базы и создали ряд высококвалифицированных промышленных институтов: ЦАГИ, ГИВД, НИФХИ им.
Л.Я. Карпова, ГИПХ и другие.
Сформировалось первое поколение советской научно-технической интеллигенции, которое ощущало свою причастность к историческим свершениям нового государства. Они создали крупномасштабную науку, великолепное образование, не уступающее западному, что облегчило нашу победу в Великой Отечественной войне и в послевоенный период способствовало решению крупных научно-технических проблем в области создания новой техники. Этот период характеризовался энтузиазмом научной молодежи и старшего поколения и коллективным творчеством, в основе которого лежали прогрессивные идеи. Мы умели делать то, что не умели делать на Западе. Мы превосходили Запад в области химической технологии (Chem. Eng. Sci.). В этом я убедился во время службы в оккупационных войсках в Германии.
Я прошел войну в составе 1-й Гвардейской танковой армии. Эта армия после войны располагалась в Саксонии (Дрезден), а командующий армией генерал-полковник М.Е. Катуков был генерал-губернатором Саксонии. Я по его поручению, как начальник Отдела штаба армии, курировал химическую промышленность и демонтаж оборудования химических заводов. Изучив химическую промышленность Германии, которая считалась лучшей в мире в довоенные годы, и её исследовательские центры, я понял, что в основе промышленной технологии лежал чисто эмпирический путь изучения процессов, осуществляемых на большой серии опытных установок. В результате созданные процессы не были оптимальными. Так, например, лучший сернокислотный аппарат, созданный Баденской анилиновой и содовой фабрикой (BASF) в Людвигсхафене мощностью 50000 т/год, состоял из трех слоев катализатора с промежуточным теплообменом и одного слоя с внутренним теплообменом, содержал 595 трубок различного диаметра. Аппарат был намного сложнее в эксплуатации и менее продуктивен, чем наши контактные сернокислотные аппараты.
Опираясь на многолетние традиции передовых ученых и инженеров нашей страны, а также учитывая принципы организации и работу Сибирского отделения РАН, нами были приняты следующие методологические и методические основы журнала:
Редакция и редколлегия журнала ставила перед собой следующие задачи:
Какие научно-технические задачи стоят перед журналом? Они определяются задачами развития промышленного катализа. Промышленные каталитические процессы многочисленны и многообразны. Поэтому в кратком выступлении невозможно перечислить многие актуальные проблемы, которым журнал должен уделять внимание. К тому же будущее российского рынка в настоящее время не имеет определенности. Поэтому необходима гибкость в определении перспектив. Для примера укажу несколько актуальных проблем, достойных обсуждения.
Создание конкурентоспособных энерго- и ресурсосберегающих промышленных каталитических процессов в следующих направлениях:
Из многочисленных проблем нашего развития я остановлюсь на трех наиболее важных из них, которые необходимы для решения этих задач:
Кинетика каталитических реакций. Кинетическая модель, как с теоретической, так и с практической точки зрения дает всю необходимую информацию для математического моделирования каталитического процесса, конструирования реактора и создания системы управления. На основе кинетической модели определяются оптимальные условия проведения процесса в промышленности, необходимое количество катализатора для достижения заданной избирательности и степени превращения, а также выбирается эффективная конструкция реактора. Однако, при существующей методике определения кинетической модели её получение является самым медленным этапом исследования. В большинстве случаев кинетическая модель реакции неизвестна, и исследователи вместе с инженерами-технологами спешат проектировать реактор интуитивно и на основе ограниченных экспериментальных измерений. Вследствие этого удлиняются сроки освоения каталитического процесса, возникает большое число ошибок и промышленность терпит значительные экономические потери. Поэтому сроки получения кинетических моделей имеют огромное практическое значение для быстрого исследования и освоения нового процесса.
Чтобы кинетическая модель могла служить опорой для решения практических вопросов, предварительная кинетическая модель должна быть получена не более, чем за месяц, а полная модель не более, чем за 4 месяца. Этим условиям должна удовлетворять техническая оснащенность и организация круглосуточных работ на нескольких установках.
До перестройки наши исследования в области кинетики реакций промышленного катализа превосходили Запад. Поэтому и математическое моделирование каталитических процессов проводилось значительно раньше и более полно, чем на Западе. В настоящее время исследования в области химической кинетики проводятся очень редко. Не организуются конференции по кинетике каталитических реакций. Напомню, что третья, и она же последняя, конференция проходила в 1992 г. в г. Иваново.
Надо понимать, что наши достижения в промышленном катализе зависят от понимания кинетики реакции промышленных каталитических процессов и надежности их кинетических моделей. Не зная кинетики реакций многих промышленных каталитических процессов, мы очень плохо характеризуем свойства промышленных катализаторов.
По характеристикам катализаторов, выпускаемых промышленностью, нельзя судить о результатах их работы в промышленных реакторах.
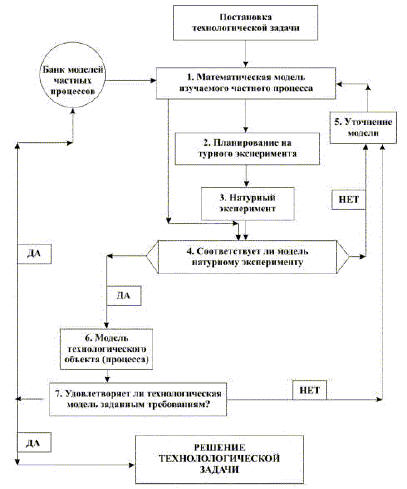
Основой методологии экспериментальных исследований каталитического процесса и кинетики реакций является сочетание натурного (НЭ) и вычислительного (ВЭ) эксперимента в автоматизированных системах научных исследований (АСНИ).
Функциональная схема АСНИ представлена на рис. 1. Структура методологии сочетания НЭ и ВЭ состоит в последовательности проведения всех этапов цикла, где каждый очередной цикл приближает к решению поставленной задачи.
Основной смысл последовательности этапов состоит в предварительном расчете и определении того, что и как надо измерять. Хотя математика стала уже неотъемлемой частью технологии каталитических процессов, необходимо дальнейшее усиление роли математики. Её значение все более возрастает в научно-техническом прогрессе промышленного катализа.
Сбалансированное сочетание вычислительного и натурного экспериментов дает нам возможность:
В большинстве случаев промышленные катализаторы содержат активные компоненты и пористую структуру наноразмеров. Однако сейчас, когда возникли инструменты, позволяющие измерять и манипулировать на наноскопическом уровне, методы нанотехнологии для получения промышленных катализаторов пробрели новое значение. Нанотехнология относится конечно не к размерам гранул катализатора, а к деталям внутренней структуры.
Молекулярная технология катализа есть научно-техническая мечта. Создаваемые путем молекулярной самоорганизации избирательные, активные катализаторы, структура которых регулируется в диапазоне атомов и молекул, является совершенно новой реальностью, которая может не иметь аналогов в природе.
Исследование каталитических систем на всех масштабных уровнях, начиная с молекулярного, требует участия специалистов многих отраслей знания. Поэтому необходим коллектив разных специалистов-единомышленников, образующий коллективный интеллект, возможности которого постоянно расширяются в отличие от индивидуального интеллекта. Как следствие, для исследования каталитических процессов необходим коллектив единомышленников, превосходящий критическую массу.
Хочу сказать несколько слов о каталитических реакторах. В ближайшее время будут широко использоваться многофункциональные реакторы с совмещенными процессами, особенно для малотоннажных каталитических процессов. Совмещение реакционных и массообменных процессов имеет целью повышение выхода целевых продуктов и уменьшение затрат энергии. Сочетание этих процессов позволяет повысить интенсивность как реакционных, так и массообменных процессов и преодолеть термермодинамические ограничения. К совмещенным относятся следующие группы процессов:
Для интенсификации этих процессов найдут применение стационарные процессы при нестационарном состоянии катализатора и реакторы с организованной симметричной насадкой и улучшенной геометрией:
Создание многопроцессорных электронных вычислительных машин открыло возможность моделирования потоков реакционной смеси в аппаратах каталитических производств на основе уравнений Навье-Стокса. Эта область получила название вычислительной аэродинамики (Computational Fluid Dynamic – CFD).
Применение методов вычислительной аэродинамики позволит улучшить понимание работы аппаратов и реакторов и найти оптимальные геометрические формы и размеры. Результаты вычислений хорошо дополняет томография, с помощью которой можно получить изображение внутренней структуры анализируемого аппарата или реактора. В настоящее время вычислительная аэродинамика (CFD) стала новым мощным орудием инженера-химика и технолога в познании работы аппаратов каталитических процессов. Открывается возможность решать задачи, не подлежащие прямому экспериментальному решению.
Области применения вычислительной аэродинамики (CFD):
Заключение
С точки зрения инновационного развития последние 15 лет были потеряны. Мы наблюдаем небольшой рост продукции, но не развитие промышленности. Нам сейчас необходимо определение целей развития. Мы должны представить себе ближайшие и долговременные перспективы в области промышленного катализа.
Настал момент, когда необходим переход на новый, качественно другой, более высокий интеллектуальный уровень на всех этапах создания каталитического процесса. У нас нет ни времени, ни ресурсов для реализации эмпирического метода проб и ошибок. Этот путь сейчас для нас губителен. Наша предпринимательская элита пребывает, в значительной мере, в плену Запада. Недооценка интеллектуального потенциала наших специалистов ставит под угрозу наши надежды на скорый выход России из глубочайшего кризиса и содействует агрессивному поведению зарубежных фирм. Например, иностранные фирмы, торгующие катализаторами крекинга, рассматривают наш внутренний рынок как свою вотчину.
Мы должны понимать, что самый короткий путь создания конкурентоспособного каталитического процесса – это путь, основанный на детальном знании всех сторон процесса, а это означает, что мы должны принять методические, технические и организационные мероприятия по сокращению сроков исследования и разработки каталитических процессов.
Большие компании и фирмы должны научиться для своего же блага опираться на знания. Экономика и техника, опирающиеся на Знания – это современный этап развития общества. Рынок знаний наиболее динамично развивается и носит глобальный характер. Инвестиции в знания должны расти быстрее, чем инвестиции в основные фонды промышленности. Богатство в интеллектуальной деятельности, а не в недрах, определяют современный этап развития страны. Руководители фирм должны понимать, что мы соревнуемся с фирмами, имеющими нарастающий опыт в течение столетий (Дюпон – 200 лет, Мобил – 150 лет), тогда как мы с энтузиазмом разрушили и, в значительной мере, потеряли собственные школы, опыт и знания. Нам многое необходимо строить заново. Поэтому нам необходимо резко увеличить капиталовложения в получение знаний, в том числе и в журнал. Это надо делать срочно, пока ещё не всё потеряно. Недостаточное финансирование науки создает условия для её уничтожения.
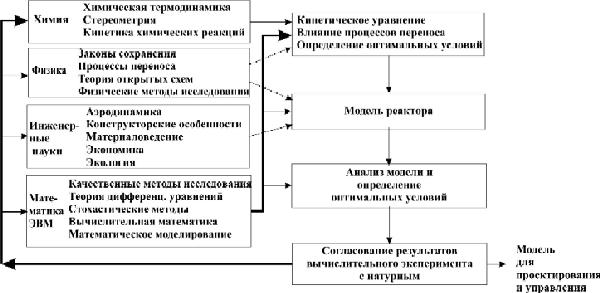
Структура методологии получения математической модели каталитического реактора
Научные основы химической технологии включают 4 блока научных дисциплин: химию, физику, инженерные науки, математику на базе современных ЭВМ. На рис. 2 стрелками показано участие и роль отдельных блоков на различных этапах исследований.
Нелинейная динамика, как эффективный междисциплинарный подход, выходит на первый план. Пройденный ею путь уже сейчас позволяет более просто и эффективно анализировать и решать задачи промышленного катализа.
Мы надеемся, что в дальнейшей работе журнала и в определении его научно-технической политики будут участвовать более широкие круги специалистов, предпринимателей и представителей фирм, что будет способствовать развитию отечественного промышленного катализа. Опираясь на самоотверженный труд наших специалистов, мы преодолеем всю меру несчастий, легших на плечи нашего народа.
М.Г. Слинько
Одной из первых в составе образованного в октябре 1958 г. Новомосковского филиала Государственного института азотной промышленности стала лаборатория физико-химических исследований. В начале 70-х годов численность Новомосковского филиала ГИАП составила почти 2000 человек. Основной специализацией института стали катализаторы.
1 апреля 1966 г. в составе физико-химической лаборатории был создан сектор исследования катализаторов, адсорбентов, реакций (ИКАР). Далее на базе сектора была создана новая лаборатория и крупный отдел исследования катализаторов (ФХИ).
Организатором и научным руководителем этих подразделений стал Голосман Евгений Зиновьевич. Сектор, лаборатория, отдел пополнялись молодыми специалистами, окончившими ВУЗы Москвы, Ленинграда, Тулы, Иваново, Воронежа, Новомосковска.
В период 1980-1991 г. в составе отдела было 3 лаборатории, а численность отдела составляла 80 специалистов. Отдел был оснащен современными приборами и установками (дифрактометры, спектрометры, дериватографы, электронные микроскопы, масс-спектрометры, установки измерения поверхности, пористости, оценки активности, измерения механической прочности катализаторов и др.). Разрабатывались и совершенствовались методы исследований катализаторов и носителей (термохроматографическое исследование процессов прокаливания и восстановления катализаторов, каталитической активности, удельной поверхности, модернизация высокотемпературной камеры для рентгенофазового анализа и др.). Среди применяемых в отделе методов, наиболее информативным является метод рентгенофазового анализа, высокий уровень применения которого в течение многих лет поддерживается высококлассными специалистами Боевской Е.А.,
Мамаевой И.А., Кругловой М.А. Неоценимый вклад в развитие метода внесли Греченко А.Н. и Клюев С.Д. В развитие метода ИК-спектроскопии для исследования катализаторов, сырьевых компонентов, механизма каталитических реакций значительный вклад внесли Саломатин Г.И., Ярошенко М.П., метода электронной микроскопии неоценим вклад Евглевского Г.М. и Кучумова В.И. Отдел посещали специалисты ведущих научных центров из многих городов нашей страны и из-за рубежа.
Сотрудники НИАП участвовали в освоении технологии производства катализаторов, разработанных с нашим участием на крупнейшей в Европе Дорогобужской катализаторной фабрике, Куйбышевском катализаторном заводе, катализаторных производствах в Северодонецке, Гродно, Кемерово, катализаторном производстве в Иране.
За 40 лет в отделе выполнили научно-исследовательские дипломные работы более 200 студентов из МГУ им. Ломоносова (Москва), ЛТИ им. Ленсовета (Санкт-Петербург), Ереванского университета, Ивановского химико-технологического института и, конечно, из НИ РХТУ им. Менделеева и Новомосковского химического колледжа.
Можно также отметить, что более половины сотрудников отдела - это бывшие студенты-дипломники и аспиранты.
Большим достижением специалистов отдела явилась фуíäаментальная разработка физико-химических основ приготоâëения ряда промышленных цементсодержащих катализаторов для важнейших процессов химической, нефтехимической, метааллургической, машиностроительной и др. отраслей.
Была создана малоотходная технология синтеза промышленных цементсодержащих катализаторов, на основе которой произведено несколько тысяч тонн никелевых, медных, циíêовых, кобальтовых, марганцевых и др. цементсодержащих катализаторов для широкого круга процессов органического, неорганического и экологического катализа. Катализаторы внедрены на более чем 170 заводах и организациях России, СНГ и дальнего зарубежья.
Высокому уровню разработок способствовали глубокие научные исследования, проводимые в отделе в содружестве с ведущими академическими и отраслевыми институтами страны, защита по изучаемым проблемам большого количества кандидатских и докторских диссертаций по исследованию и синтезу цементсодержащих катализаторов и носителей. Это диссертации сотрудников отдела (Голосмана Е.З., Ефремова В.Н., Саломатина Г.И., Греченко А.Н., Григорьева В.В., Бельмеса М.Н., Караманенко С.В., Турченинова А.А., Обысова М.А.) и сотрудников ИОХ им. Зелинского (Якерсона В.И., Дулова А.А., Абрамовой Л.А., Соминского О.Д., Дых Ж.Л., Брук И.А., Франкфурта Г.И., Ибраевой З.А., Ниссенбаум В.Д., Лепского В.И.), сотрудников МИТХТ им. Ломоносова (Антонюка С.Н., Егоровой Е.В.), сотрудников МГУ (Ткаченко С.Н., Саблуковой И.В., Махова Е.А., Мартынова И.П.), сотрудников РХТУ и НИ РХТУ им. Менделеева (Бреевой Н.В., Моисеева М.Н., Таратухина А.В. и др.), ИНХС им. Топчиева РАН (Закировой А.Г., Закорчеваной Ю.П.), сотрудников Ереванского Госуниверситета (Мушегян Н.А.), сотрудников Университета нефти и газа (Осман Бурхан и др.), ЛТИ им. Ленсовета (Бельмеса М.Н.).
Все темы научно-исследовательских дипломных и диссертационных работ связаны с разрабатываемым с 1968 г. направлением: разработка и исследование высокоэффективных катализаторов на основе специальных особо чистых цементов.
Наиболее тесное содружество (с 1966 г.) "ИКАРА", а затем отдела (ФХИ) возникло с лабораторией исследования катализаторов Института органической химии им. Зелинского - ИОХ (Якерсон В.И., Рубинштейн Л.М., Дулов А.А., Клячко А.Л., Лафер Л.И., Данюшевский В.Я., Слинкин А.А., Акимов В.М., Ниссенбаум В.Д., Абрамова Л.А., Капустин Г.И.,Шашков Д.П., Соминский С.Д., Субботин А.Н. и др.)
Далее содружество продолжилось и с другими лабораториями ИОХ: (Лапидус А.Л., Крылова А.Ю., Брук И.А., Франкфурт Г.И., Синева Л.Ю. и др.); группа каталитического синтеза (Исагулянц Г.В., Беломестных Д.П.); группа кинетики каталитических процессов (Киперман С.Л., Некрасов Н.В., Гудков Б.С., Гайдай Н.А.); лаборатория катализа на редких и рассеяных элементах (Миначев Х.М., Шпиро Е.С., Усачев Н.Я. и др.); лаборатория радиоспектроскопических и оптических методов изучения механизма гетерогенного катализа (Казанский В.Б.).
Итогом многолетних исследований стали разработки высокоэффективных катализаторов метанирования (НКМ-4А, НКМ-2А), получения защитных атмосфер (КДА), синтеза бутиловых спиртов (НТК-10), сероочистки увлажненных газов (ЦКА), получения искусственных бензинов (КЦ), стирола, очистки газов от кислорода (НКО) и др.
Впервые открыт эффект низкотемпературного (280 - 400 oС) разложения карбоната кальция в составе никелевых, медных, кобальтовых и др. цементсодержащих катализаторов в восстановительных средах и предложен механизм разложения кальцита (Греченко А.И., Ефремов В.Н., Караманенко С.Н., Якерсон В.И.).
Многие из разработанных катализаторов внедрены в различные отрасли промышленности. Совместно опубликовано около 200 статей и тезисов докладов, брошюр, получено несколько десятков патентов на изобретения.
Опубликованы монографии по проблеме цементсодержащих катализаторов Голосман Е.З., Якерсон В.И. "Производство и эксплуатация промышленных цементсодержащих катализаторов". Черкассы, НИИТЭХИМ, 1992, 434 с. и Якерсон В.И., Голосман Е.З. "Катализаторы и цементы", М.: Химия, 1992, 256 с.; Голосман Е.З. Промышленные катализаторы на основе специальных цементов для интенсификации технологических процессов и обезвреживания отходящих газов. М.: ЦНИИТЭнефтехим, 1997, 44 с.
Работы с различными лабораториями ИОХ им. Зелинского продолжаются. Это и создание, и исследование катализаторов синтеза метиланилина, конверсии толуола, исследование критических явлений в гетерогенном катализе (Мортиков E.С., Бачурихин А.Л., Кустов Л.М., Стахеев А.Ю., Гудков Б.С., Субботин А.Н. и др.).
Большие совместные работы в области физико-химической механики были осуществлены сотрудниками отдела к.х.н. Греченко А.Н., Нечуговским А.И. и др. со специалистами отдела института физической химии РАН (Щукин Е.Д., Конторович С.И., Бессонов А.И.). На основе представлений физико-химической механики была создана серия высокопрочных катализаторов НКМ-2А и др., а серия катализаторов для низкотемпературной конверсии оксида углерода получила аббревиатуру ФХМ (физико-химическая механика). Это промышленные катализаторы серии НТК-10 (ФХМ), загруженные в десятки конверторов (с объемом загрузки до 100-150 тонн в один конвертор) больших агрегатов синтеза аммиака и в Горловке, Новомосковске, Северодонецке, Черкассах, Магнитогорске, Нижнем Новгороде, Кирове, Мажейкяе.
Многолетние исследования по изучению и разработке катализаторов получения формальдегида, очистки газов от низкомолекулярных меркаптанов проводились с химфаком МГУ им. Ломоносова (Грязнова О.В., Иванов В.А., Борисенкова С.А. и др.). В настоящее время сотрудниками отдела Кругловой М.А., Сатановским Ю.И., Козыревой Г.В., Козловой И.В. активно проводятся совместные исследования с учеными МГУ по очистке газов от озона, каталитической очистке шахтного метана (Лунин В.В., Ткаченко С.Н., Егорова Г.В., Зосимов А.В.). Можно особо отметить, что, например, водостойкие, механически прочные, активные катализаторы разложения озона внедрены более, чем на 20 предприятиях химической, оборонной, пищевой и др. отраслях России, СНГ, Таиланде, Швейцарии.
Крупные работы по синтезу катализаторов и их испытаниям осуществлялись специалистами НИАП Ефремовым В.Н., Трошиной В.А., Дульневым А.В., Тесаковой Г.М., Ткановой Л.И., Гельманом В.Н. и др. с рядом организаций по очистке технологических и выбросных газов от оксидов азота, углерода, аммиака, стирола, фенола, ацетона, крезольных лаков, кумола, циклогексана. Это, прежде всего ВНИИОС НК (Саблукова И.В.), концерн по производству полиуретанов - Нижний Новгород - (Дергунов Ю.И.), ГИАП, НИ РХТУ, Авиапром, "Микропровод", НИПИМ Тула (Хейфец В.И.), завод автомобильных катализаторов - Новоуральск (Порсин А.В.), Чебоксарский кабельный завод.
К компонентам, представляющим большой интерес, относится аллен - весьма перспективный продукт органического синтеза с широкой областью применения: стабилизаторы лаков, присадки к маслам, резина, полимерные материалы, мономеры и др. При исследовании разработанных цементсодержащих носителей галюминов в качестве катализаторов процесса изомеризации метилацетилена в аллен совместно с ВНИИОС Москва показана возможность их применения на образцах характеризующихся определенным соотношением кислотных и основных центров (Табер А.М., Гузенко Л.К., Черных С.П.).
Совместно с институтом ВНИИСинтезбелок (Москва), ИОХ им. Зелинского были созданы устойчивые к жидкофазной среде катализаторы серии ВИГ гидрогенолиза глюкозы для получения глицерина и гликолей (Полетаева Т.И.). С кафедрой ОХТ Казанского технического Университета проводятся работы по созданию катализатора гидрирования ацетофенона (Харлампиди Х.Э., Каралин Э.А.). Совместно с ВНИИНЕФТЕХИМ (Санкт-Петербург) проводились и осуществляются работы по созданию катализаторов синтеза анилина, получения g -бутиролактона, 2-этилгексанола, дожига газов производства изопрена (Якушкин М.И., Хворов А.П., Пастор В.Е.). Ряд из разработанных катализаторов прошли промышленные испытания на Новочеркасском заводе синтетических спиртов, Омском заводе "Химпром", Волжском "Нефтеоргсинтез". С институтом двигателей НИИД, Ереванским и Саратовским университетами проводились работы по синтезу оксидных никельмедных, никельмедьмарганцевых, никельмедьпалладиевых катализаторов очистки выбросных газов двигателей внутреннего сгорания (Смирнова Т.Н., Болдырев И.В., Мушегян А.В., Кузьмина Р.И., Севостьянов В.П.).
Значительные исследования по разработке катализаторов метанирования, разложения и синтеза аммиака, конверсии оксида углерода, дегидрирования циклогексанола, очистки газов производства капролактама, исследованию катализаторов методами РФЭС и др. проводились с Московским ГИАП (Алексеев A.M., Березина Ю.И., Геминова М.И., Дьяконов Я.И., Зозуля В.Ю., Клинова Л.Л., Полевой А.С., Проскурнин А.М., Рабина П.Д., Семенова Т.А., Чеснокова Р.В., Чудинов М.Г., Шполянский М.А. и др.).
С Институтом катализа СО РАН (Новосибирск) был выполнен цикл работ по исследованию катализаторов на основе алюминатов кальция методами ЯМР27 Al высокого разрешения и кислотно-основных свойств алюмокальциевых катализаторов, носителей и адсорбентов (Мастихин В.М., Паукштис Е.А., Юрченко Э.Н.), осуществлена оценка активности катализаторов ГТТ, КЦ, НТК-10 в процессе тонкой очистки азотоводородной смеси от примеси оксидов углерода и в процессах селективного окисления аммиака для обезвреживания газовых выбросов (Садыков В.А., Носков А.С., Боброва Л.Н.). Можно выразить глубокую признательность академику Пармону В.Н., чл.-корр. РАН Буянову Р.А., профессору Носкову А.С., Старцевой Л.Я., Замулиной Т.В. и многим другим сотрудникам Института Катализа за приглашение наших специалистов для участия в прекрасно организованных научных конференциях в Новосибирске, Минске, Ярославле, Стерлитамаке, Омске, Владимире, Санкт-Петербурге и др. по исследованию и приготовлению катализаторов.
Созданию и внедрению катализаторов серии НКО-2 очистки технологических газов от кислорода и метана вместо палладиевых (АПК-2) были посвящены работы с Балашихинским "Криогенмаш", п/о "Хром" (Файнштейн В.И., Савинов М.Ю. и др.). Катализаторы внедрены на ряде заводов в Норильске, Новокузнецке, Муроме, Новомосковске, Череповецке, Мариуполе, Новолипецке, Полевском, Нижнекамске, Узбекистане, Киргизии. Катализаторы эксплуатируются в процессе очистки аргона, азота, криптон-ксеноновых смесей и обеспечивают очистку этих газов от CH4 (ниже 1 ppm) и О2 (ниже 5 ppm). Сверхтонкую очистку газов от кислорода (ниже 1 ppm) обеспечивают разработанные хемосорбенты НКО-3Х. Промышленное внедрение эффективных катализаторов очистки выбросных газов производств азотной кислоты от оксидов азота, аммиака, оксида углерода проводилось на Кемеровском, Березниковском, Менделеевском, Новомосковском п/о "Азот". Кировочепецком химкомбинатах (Ефремов В.Н.. Тесакова Г.М., Зиновьева Т.А., Дульнев А.В.)
Созданию и исследованию формирования катализаторов хлорорганического синтеза (получение винилхлорида, аддитивное окислительное хлорирование этилена и др.) были посвящены и продолжаются работы с ГОСНИИХЛОРПРОЕКТ (ныне НИИ "СИНТЕЗ") (Курляндская И.А., Глазунова Е.Д., Соломоник И.Г., Трегер Ю.А., Флид М.Р.). Применение этих катализаторов, например, в производстве винилхлорида позволит сократить объем хлорорганических отходов, исключить выброс в атмосферу оксидов углерода и серы, снизить энергетические затраты, повысить сбалансированность процесса по хлору. Промышленные испытания одного из катализаторов проводились на Усольском химкомбинате.
Большой пласт исследований был осуществлен совместно с МИТХТ им. Ломоносова, ИОХ им. Зелинского по созданию катализаторов разложения метанола (с целью получения защитных и восстановительных атмосфер и очистки выбросных газов), превращения метанола и воднометанольных смесей (Антонюк С.Н., Егорова Е.В.и др.).
Полученный после разложения воднометанольной смеси газ может быть использован в качестве эффективного альтернативного топлива для двигателей внутреннего сгорания (ДВС), а газ, полученный после разложения метанола, используется в качестве контролируемой атмосферы для предотвращения окисления и изменения химического состава поверхности металла и др. целей.
С институтом Газа (Киев) проводились исследования по применению катализаторов КДА генерации водорода для топливных элементов (Стеженский А.И. и др.).
Обысовым М.А., Ефремовым В.Н. совместно с Тульским университетом выполнены и продолжаются работы по механоактивации катализаторов с использованием воды высокого давления (Бреннер В.А., Жабин А.Б., Пушкарев Е.А., Головин К.А.). С институтом органического катализа и электрохимии (ИОКЭ) Алма-Ата осуществлялись исследования катализаторов получения циклических спиртов, синтеза d, l ментола и катализаторов на базе активированных алюминиевых сплавов (Сармурзина Р.Г., Конуспаев С.Р.). Очистка вентиляционных выбросов и других газов от низкомолекулярных меркаптанов и окислительная демеркаптанизация нефтяных фракций на фталоцианиновых цементсодержащих катализаторах проводилась с НИОПИКом, МГУ им. Ломоносова, ВНИИОС НК, ВНИУС ((Казань) (Калия О.Л., Мазгаров А.М., Вильданов А.Ф., Саблукова И.В., Борисенкова С.А.). Отработана технология приготовления катализаторов с использованием цементов и галюмина в форме таблеток и колец. Одна из модификаций катализатора ФК прошла пилотные испытания на Волжском заводе органического синтеза в процессе обезвреживания метилмеркаптансодержащих вентиляционных выбросов. Концентрация метилмеркаптана на входе в реактор колебалась от 5 до 50 мг/м3, а на выходе из реактора не превышала 0,05 мг/м3.
При дефиците нефтепродуктов и возрастающей потребности в моторных топливах весьма актуальной становится переработка тяжелых нефтяных остатков. В результате проведенных совместно с ИНХП (Баку) исследований (Ахвердиев Р.Б.) показана возможность применения модернизированных катализаторов серии НКО-2-3. Разработанный катализатор способствует повышению выхода светлых нефтепродуктов и одновременно обеспечивает облагораживание мазута за счет уменьшения содержания асфальтосмолистых и коксующихся веществ.
Совместно с ИНХС РАН (Москва) проводятся работы по исследованию и получению катализаторов очистки газов от оксидов азота, углеводородов (НТК-10-1) и каталитических композиций для синтеза диметилового эфира v экологически чистого топлива (Розовский А.Я., Лин, Г.И., Третьяков В.Ф., Бурдейная Т.Н.)
Крупномасштабное внедрение катализаторов низкотемпературной конверсии оксида углерода, метанирования в агрегатах синтеза аммиака, очистки выбросных газов от оксидов азота осуществлялось на ряде заводов СССР, России и СНГ и в том числе на Новомосковской акционерной компании "Азот". Работа проводилась совместно со специалистами комбината (Воробьев B.C., Воробьев В.Ф., Дробязко А.И., Ежов В.А., Ильичев Н.Ф., Корнев А.Г., Копылов А.В., Поливанов Б.И., Самарин В.Н., Тарасов Е.H., Тарев В.В., Тихомиров В.Е., Хилкова А.А., Худолей В.А., Шуляковский A.M.). В настоящее время сотни тонн катализаторов серии НТК-10 эксплуатируются в крупнотоннажных агрегатах синтеза аммиака НАК "Азот", Черкасского ПО "Азот", в конверторах электротехнического завода "Новая Этна" и других. Высокопрочные активные катализаторы серии НТК-10 демонстрируют высокую устойчивость к капельной влаге, обладают большой серостойкостью и хлороустойчивостью. Это позволило предложить один из катализаторов этой серии (НТК-10-2ЛФ) в качестве "лобового" слоя при применении практически любых катализаторов низкотемпературной конверсии оксида углерода. Хорошие результаты демонстрируют катализаторы очистки выбросных газов от оксида углерода на Кемеровском п/о "Азот" (Царицын С.Н., Кононова И.Н., Бычкова Г.И.). Высокоэффективные, термостабильные, высокопрочные катализаторы метанирования НКМ-4А эксплуатировались и эксплуатируются в 11 метанаторах больших агрегатов различных химических комбинатов, обеспечивая очистку от СО и СО2 ниже 5v10 ppm в течение всего срока эксплуатации. Особо можно отметить фантастический срок службы этих катализаторов - 15-16 лет.
Благодаря тщательной изученности, длительным предварительным пилотным испытаниям на реальных газах эти катализаторы являются одними из самых лучших в мире. Несмотря на экспансию импортных катализаторов, доля отечественных в процессе метанирования и в настоящее время в отличие от очень многих катализаторов, составляет около 100%.
С Институтом атомной энергии им. Курчатова (Столяревский А.Я.) и технологическим отделом НИАП (Тительман Л.И.) были проведены испытания высокотемпературного катализатора метанирования НКМ-2А для процесса "Адам и Ева" (система дальнего теплоснабжения на базе высокотемпературного ядерного энергоисточника, использующая обратимые химические реакции конверсии метана водяным паром и синтеза метана из оксидов углерода и водорода). Разработанные катализаторы сохраняют высокие механические свойства, термостабильность и активность в течение длительного пробега, не подвергаются зауглероживанию.
Крупные совместные работы по созданию и внедрению катализаторов синтеза бутиловых спиртов были проведены с ПО "Салаватнефтеоргсинтез" (Павлычев В.Н., Евдокимова Ж.А. и др.). Было наработано несколько десятков тонн катализаторов для этого процесса НТК-10-1, НТК-10-1 ФХМ. Процесс синтеза (через альдегид) протекает при достаточно высоких давлениях (280-350 атм.) в присутствии воды в газе, т.е. необходим катализатор, работающий при достаточно жестких условиях. При эксплуатации в течение более 10 лет разработанных катализаторов в 30 и 15 тонных реакторах (по объему катализаторов) были выявлены следующие преимущества по сравнению с ранее использовавшимися катализаторами: более высокая степень гидрирования масляных альдегидов и селективность по спиртам, сокращение времени восстановления с 7 до 3-4 суток, сокращение расхода топливного газа (в 2-2,5 раза), более мягкие условия гидрирования v 200-250 oС, вместо 280-320 oС.
Совершенствованию цемента марки талюм посвящены работы с НИИЦЕМЕНТ (Подольск) и кафедрой цементов РХТУ им. Менделеева (Москва) (Кузнецова Т.М., Гумаров Р.Х., Орлов В.Я.).
Созданию специальных особо чистых цементов марки талюмин для производства катализаторов были посвящены работы с Челябинским "УРАЛНИИСТРОМПРОЕКТ" и комбинатом редких металлов (Чорух Дайрон, Таджикистан)
(Залдат Г.И., Егоров О.Г., Таджиев В.Я. и др.). Была синтезирована крупная промышленная партия этого цемента и на основе талюмина приготовлены опытно-промышленные партии катализаторов.
Переработке шлаков алюминотермической плавки феррохрома и ферротитана для приготовления катализаторов и цементов были посвящены работы Боевской Е.А.,Мамаевой И.А. и др. с сотрудниками Ключевского завода ферросплавов (Гильварг С.И., Казакевич В.И.).
Современные химические производства, порошковая металлургия, изготовление электротехнических, машиностроительных и других видов сталей, кинескопов, медицинских инструментов, ювелирных изделий, специальных видов стекла и др. требуют применения защитных (контролируемых) атмосфер. Контролируемые атмосферы - это газовые среды, для которых характерно направленное взаимодействие с поверхностным слоем металла при его термической обработке. Одним из наиболее экономически оправданных путей получения этих атмосфер является каталитическое разложение аммиака. Например, в США на эти цели используются сотни тысяч тонн аммиака в год. Важным направлением является использование на объектах, не оснащенных источниками водорода крекинга, газообразного аммиака для получения азотоводородной смеси, которая применяется для восстановления катализаторов агрегатов синтеза аммиака большой единичной мощности в период пуска.
Около 100 заводов и организаций в России, СНГ и дальнем зарубежье используют разработанные высокотемпературные катализаторы серии КДА для получения защитных атмосфер и восстановительных сред. Срок службы этих катализаторов составляет 15 лет и более. Среди пользователей катализаторов КДА Новолипецкий, Белорецкий, Магнитогорский металлургические комбинаты, металлургические заводы в Индии, Болгарии и Пакистане, Первоуральский, Волгоградский трубные заводы, Пермский велосипедный, Казанский авиационный, Санкт-Петербургский завод "Русские самоцветы", Ярославский моторный, Молодеченский завод порошковой металлургии, Рязанский завод счетно-аналитических машин, Волжский автомобильный, Днепродзержинское и Одесское ПО "Азот".
Промышленное производство этих катализаторов освоено катализаторным производством НИАП, одной из марок КДА на Гродненском ПО "Азот" (Короткий И.П., Ницкая В.Н., Юрша И.А.).
Перспективным направлением является разработка модифицированных высокотемпературных катализаторов КДА для очистки коксовых газов от аммиака и сернистых соединений при температурах 1100 - 1150 0С. Работа проводится с институтом ВУХИН (Екатеринбург), Гипроникель (Санкт-Петербург) v Платонов О.И., Сауль О.П., Андрейков Е.И. Для различных процессов очистки газов проверку катализаторов осуществляли и в Дзержинском НИОГАЗ (Лиманский Г.М. и Торопкина Г.Н.).
Большая помощь оказывалась и оказывается отделу ФХИ НИАП различными кафедрами НИ РХТУ им. Менделеева (Новомосковск). К работе, как указывалось, привлекаются дипломники, аспиранты и преподаватели. Это, прежде всего кафедра технологии неорганических веществ, кафедра технологии основного органического и нефтехимического синтеза, кафедра химической технологии керамики и огнеупоров. Это исследования цементсодержащих носителей, модернизация катализаторов конверсии оксида углерода, гидрирования жиров, очистки газов от оксидов азота и низкоконцентрированного аммиака, а также утилизации оксидов азота на специально подготовленном фториновом волокне (Леонов В.Т., Моисеев М.М., Лебедева Г.Ф., Крутов Ю.А., Крутова В.П., Ефремов А.Н., Иконников Н.К.).
С РХТУ им. Менделеева (Москва) проводятся исследования катализаторов очистки газов, работы по созданию пеноподобных керамических материалов (Бесков B.C., Вишняков А.В., Чащин В.А., Клушин В.Н.). В экспериментах, проведенных ВНИНЕФТЕХИМ (Бельмес М.Н.) на установках комбината "Киришинефтеоргсинтез", подтверждена высокая активность медьцинкалюмокальциевых и медьцинкникельмарганцевых цементсодержащих катализаторов в процессе очистки газов от оксидов азота при использовании в качестве восстановителя водорода, оксида углерода.
Разработка катализаторов гидрогенизации сложных эфиров дикарбоновых спиртов проводилась с ГОСНИИМетанолпроект (ГОСНИИТехнология - Северодонецк) (Воронков А.П.). Исследованию синтезированных катализаторов парофазного гидроаминирования фурфурола и гидрогенолиза фурфуриламина, конверсии нафты для других процессов осуществлялись ВНИИХТИМП - Ташкент (Махкамов Х.М., Воробьев В.Н., Молодоженюк Т.Б.) Плазмохимический синтез активных окислов металлов, моноалюминатов кальция, радиационный синтез оксидов азота осуществлялся с отделом плазмохимии НИАП (Горожанкин Э.В., Шенкин Я.С., Левченко В.Ф. и др.). Многие из работ, проводимых отделом ФХИ осуществляются со специалистами технологических и др. лабораторий и отделов НИАП (Галкина А.И., Данциг Г.А., Дронов А.Е.,Ефремов В.Н., Козлов Л.И., Крейндель А.И., Левицкая Н.А., Левченко В.В., Лыткин В.П., Мурашов Н.И., Обысов А.В., Селютина М.Г., Соболевский В.С., Старовойтова Г.А., Сокова Е.Е., Турченинов А.Л., Чуприн В.М., Шаркин Г.А., Ягодкин В.И.).
Из защит кандидатских диссертаций последнего времени можно отметить высокий уровень работ, представленных сотрудниками отдела, выпускниками кафедры ТНВ НИ РХТУ им. Менделеева Веры Трошиной и Алексея Дульнева. О научном потенциале отдела свидетельствуют более 500 научных статей и тезисов докладов, несколько монографий и брошюр.
В разные годы разработки отдела выставлялись на крупных зарубежных выставках Франции, Германии (Ганновер и Лейпциг), Венгрии (Будапешт), Болгарии (Пловдив), Сирии (Дамаск), Югославии (Загреб), Австрии (Вена), на выставках в Москве Химия 2001-2005 гг., ВДНХ.
Получены около 100 Российских и международных патентов. На все разработанные промышленные катализаторы подготовлены и утверждены технические условия (Чкунина Т.П., Королева Л.М.), выданы рекомендации по загрузке, восстановлению, эксплуатации катализаторов. Сотрудники отдела ежегодно выступают на Российских и международных конференциях. Постоянной трибуной для выступлений научных сотрудников, преподавателей, инженеров города является также созданный главным образом на базе отдела и кафедры ТНВ НИ РХТУ городской научно-практический семинар "Катализ. Катализаторы. Охрана окружающей среды" (научные руководители семинара профессор Голосман Е.З. и доцент Леонов В.Т.), в котором принимают участие заводы, НИИ, ВУЗы, химический колледж г. Новомосковска. Отдел являлся одним из основных организаторов ежегодных крупных научных конференций проходивших в НИАП.
Нельзя не вспомнить и участие сотрудников отдела в спортивных соревнованиях, КВН города. В течение многих лет отдел выставлял для участия в крупнейших Всесоюзных, Российских соревнованиях команду блестящих спелеологов во главе с основателем и руководителем городской секции спелеологии, инструктором высшей категории Греченко А.Н. (Ярошенко М.П., Быкова Е.Л., Мамаева И.А., Клюев С.Д., Бельмес М.Н., Архипов С.Н., Растегаев О.В. и др.). А дружба и капустники с Новомосковским театром (Таршис С.А., Рудник Д.), поездки на велосипедах на Голубое озеро, выезды на Оку, Пронь, Волгу.
Специалисты отдела активно участвуют в общественной жизни города, области, страны. Это - научные общества, редколлегия журнала ("Катализ в промышленности"), диссертационные ученые Советы РХТУ им. Менделеева, Тульского госуниверситета. Выступают по радио, телевидению, готовят публицистические статьи для Российских газет и журналов.
Ряд специалистов, выросших в отделе, пошли, как говорится, на повышение - заместителем директора по науке стал к.т.н. Ефремов В.Н., зав. лабораторией - к.т.н. Дульнев А.В., начальником отдела - к.т.н. Обысов М.А.
Сегодня численность отдела сократилась более чем в 2 раза. Отдел состоит из двух лабораторий: физико-химических исследований и очистки технологических и выбросных газов. В последние годы лаборатория пополнилась молодыми сотрудниками, выпускниками НИ РХТУ, МГУ, ряд из которых стали и становятся квалифицированными, ведущими специалистами (Круглова М.А., Козлова И.В., Виноградова Е.Н., Козырева Г.В., Пронина Е.А. и др.). Конечно, становление молодых ученых - это сложный и длительный путь. Нужна помощь, и прежде всего, компании "Алвиго" (Председатель совета директоров Полозов В.А.), в состав которой сейчас входит и НИАП. Нужны новые приборы и установки, современные компьютеры и, конечно, достойная зарплата.
Отдел ФХИ участвует в разработке технологии формованных катализаторов метанирования, разложения озона, конверсии оксида углерода с размером гранул от 1,0 до 5,0 мм, проводятся работы по созданию нового поколения катализаторов гидрирования жиров. Проводятся исследования по синтезу высокоэффективных катализаторов гидрирования оксидов углерода различной геометрической формы с пониженным содержанием активного компонента, получению оксидных катализаторов с добавками минимальных количеств драгметаллов, синтезу катализаторов устойчивых для эксплуатации в жидкофазной среде. Промышленное производство всех разработанных цементсодержащих катализаторов освоено катализаторным производством НИАП (Пуклик И.Р., Вейнбендер А.Я., Головков В.И., Шкитина В.И. и др.). Новые технологии использованы специалистами ряда НИИ, ВУЗов в России, СНГ при создании цементсодержащих каталитических композиций, а так же специалистами катализаторных производств Дорогобужа, Северодонецка, Гродно и др. (Фирсов О.П., Павелко В.З., Калинченко Ф.В., Юрша И.А., Ницкая В.И., Короткий И.П. и др.).
Совместно со специалистами "АЛВИГО-М" ведутся работы по модернизации катализаторов низкотемпературной конверсии оксида углерода на основе сложных медьцинковых солей, дегидрирования циклогексанола, очистки газов, синтеза ряда органических соединений (Крейндель А.И., Данилова Л.Г., Комова З.В., Бруштейн Е.А., Калиневич А.Ю., Шустов В.А., Довганюк В.Ф.).
Упомянуть все разработки отдела, всех создателей и исследователей катализаторов и носителей невозможно. За 40 лет в отделе в разные годы поработали около 130 сотрудников и аспирантов. Обо всех совместных работах с коллегами из различных лабораторий, отделов научной и проектной части НИАП, катализаторного производства, НИИ, ВУЗов, заводов можно вспоминать только с большой благодарностью. И, конечно, особую благодарность и низкий поклон хотелось бы передать профессору Владимиру Ильичу Якерсону, нашему другу, коллеге, наставнику многих сотрудников отдела, все 40 лет прошагавшему вместе с "ИКАРОМ" и давшему всем нам вводную в катализ, в методы, в умение писать статьи, диссертации, полемизировать на конференциях и защитах.
История "ИКАРА" запечатлена помимо публикаций, патентов, диссертаций в десятках фотоальбомах с юмористическими подписями и стихами и видеофильмах, хранящихся в отделе.
40 лет - это, как говорится, время свершений. Кое-что было сделано, но надеемся, несмотря на наше трудное время, сотрудникам юбилейного отдела многое еще удастся свершить и совершить.
Зав. отделом НИАП, д.х.н., профессор,
Заслуженный химик России
Голосман Е.З.
Недавно в Туле объемом 260 стр. издана книга "Научный потенциал Тульской области". Издание подготовлено Тульской областной универсальной научной библиотекой (ОУНБ) (авторы-составители - Королева Л.И., Гридчина Ю.В., Дементьева А.А., Колпакова Е.В.), комитетом Тульской области по науке (проф. Шадский Г.В., Белов А.М.). Помощь в сборе данных оказал и Союз научных и общественных организаций Тульской области (проф. Чуков А.Н. и проф. Сладков В.Ю.). Книга подготовлена при поддержке гранта губернатора Тульской области в сфере науки и техники 2005г. Об уникальности издания отмечено в предисловии к книге губернатора Тульской области В.Д. Дудка.
В известной мере эта книга явилась продолжением изданной в 2000 году книги "Тульские ученые накануне третьего тысячелетия".
В книге (2000г.) помимо аналитических материалов впервые были представлены справочные сведения об отдельных ученых, специалистах и изобретателях, а также аннотированный библиографический указатель опубликованных за 20 лет (1981 - 2000 г.г.) значимых и пионерских работ.
Почти за год до издания новой книги во все организации области, ряду специалистов различных отраслей были разосланы письма и анкеты с просьбой изложить сведения о направленности работ каждого научного сотрудника и библиографии научных трудов.
Эти материалы помимо подготовки издания новой книги собирались и для продолжения базы данных "Научный потенциал Тульской области", в которой представлены сведения о более двух тысячах ученых и специалистах Тульской области в различных областях научной деятельности и пять тысяч опубликованных ими научных работ.
Новое издание книги состоит из трех основных разделов:
Как отмечено в книге, к сожалению, не все организации, особенно ВУЗы, в полной мере откликнулись на проводившееся анкетирование, в связи с чем, отдельные научные направления отражены недостаточно.
Помимо справочных и библиографических разделов, книга содержит анализ реализации региональной научно-технической и инновационной политики в Тульской области в 2000 - 2004 г.г.
Отмечено, что в сфере науки в области действуют более 40 научных организаций и около 80 малых предприятий, 12 учреждений высшей школы и их филиалов, а также научно-исследовательские и проектно-конструкторские подразделения в 62 промышленных предприятиях. В них занято более 16 тысяч человек. Непосредственно научные исследования и разработки выполняли порядка 10 тысяч человек в 26 организациях области и более 2 тысяч на инновационно активных предприятиях. За последние 4 года число специалистов с высшим и средним образованием выросло на 2%.
Основные научные силы области сосредоточены в городах Туле и Новомосковске.
Научная деятельность охватывает такие имеющие важнейшее значение отрасли народного хозяйства как оборонная, химическая, машиностроение, радиоэлектроника, добыча полезных ископаемых, медицинских технологий и оборудования и др. Из числа специалистов, занимающихся исследованиями и разработками, более 80% работают в сфере технических наук. Рассмотрим подробнее одно из направлений освещенных в справочнике. Научно-техническим обеспечением развития химической промышленности в области занимаются шесть крупных научных организаций, среди которых в первую очередь следует отметить Новомосковский институт азотной промышленности (НИАП, ген. директор Бунин И.Е.), научно-исследовательский институт мономеров (НИПИМ, ген. директор Кудашов А.А.), Тулагипрохим. Большой вклад в развитие прогресса химической индустрии вносят Новомосковский институт Российского химико-технологического университета им. Менделеева (НИ РХТУ, ректор - проф. Вент Д.П.), Тульский госуниверситет (президент - проф. Соколов Э.А.) и Тульский педагогический университет (ректор - проф. Шайденко Н.А.).
В области успешно работают гиганты химической промышленности Новомосковская акционерная компания "Азот" (ген. директор Голосниченко П.А.), Щекинское п/о "Азот" (ген. директор Сокол Б.А.), Ефремовский завод СК (гл. инженер Ряховский В.С.), Алексинский химический комбинат (ген. директор Соколов А.Г.), "Проктер Энд Гэмбл" - (Новомосковскбытхим) - (ген. директор Мищенко А.Н.), Новомосковский завод "Полимконт" (Мухин В.Г.), Щекинский завод "Химволокно" (ген. директор Махно А.А.), комбинат органического синтеза (ген. директор Федоренко В.В.) и др.
Среди специалистов отмеченных в книге химики и горняки Новомосковска: НИАП (Гартман В.Л., Голосман Е.З., Дульнев А.В., Ермина З.Е., Ефремов В.Н., Круглова М.А., Мамаева И.А., Обысов М.А., Серегина Л.К., Соболевский В.С., Шаркина В.И., Ярошенко М.П., Ягодкин В.И.), НИ РХТУ (Бабокин Г.И., Вент Д.П., Земляков Ю.Д., Кизим Н.Ф., Коробко Е.А., Лебедева Г.Ф., Лебедев К.С., Магергут В.З., Маклаков С.А., Медведев Г.И., Перегудов В.А., Рыбкина Т.И., Савельянов В.П., Сафонов Б.П., Сидорчук В.К., Филимонов В.Н., Чернышева Н.И., Янков А.В.), НАК "Азот" (Андросов П.Д., Замуруев О.В., Лонина Н.Г., Ляхин Д.В), ПНИУИ (Голоуб В.П., Потапенко В.А., Цыплаков Б.В.) и другие. Важно, что наряду с уже маститыми специалистами в справочник вошли и достаточно молодые ученые.
Наверное, полнота издания этого справочника была бы выше, если бы удалось привлечь значительно большее количество организаций и специалистов. Если бы практически каждый потенциальный участник получил персональную информацию. Необходимо анкеты и информацию о предполагаемом издании размещать в областных и городских газетах, сообщать по радио и телевидению, доводить до сведения мэрии всех городов и районов области, а также библиотек региона, научно-технических обществ СНИО. Конечно, это требует дополнительных расходов и помощи администрации области, комитета по науке. Как представляется это, безусловно, необходимо и целесообразно осуществить при новом издании книги.
Конечно столь маленький тираж книги (300 экз.) не позволит обеспечить справочником все организации, библиотеки (городские, районные, ВУЗовские, школьные, колледжей) и специалистов. Наверное, есть смысл осуществить допечатку тиража, предварительно собрав заявки на переиздание.
В книге сделан анализ научно-технического потенциала области и в том числе рассмотрены объемы выполненных научно-технических работ, численность специалистов выполнявших научные исследования и разработки, объем затрат.
Показано, что по данным Роспатента в рейтинге регионов Центрального Федерального округа Тульская область занимает четвертое место, вместе с тем, достигнутые результаты выглядят недостаточно успешно по сравнению с дореформенным периодом. Особенно заметно отставание при использовании изобретений (уменьшение в 9 раз). Причиной резкого падения количества подаваемых заявок на изобретения является неоправданная ликвидация в России (1992г.) систем бесплатного рассмотрения заявок. В книге обсуждается и организация государственной поддержки научно-технической и инновационной деятельности на территории области. К сожалению, финансирование научных исследований из областного бюджета весьма скромно.
Не менее скромно финансирование и из федерального бюджета. Затраты на науку в России в сопоставимых ценах - менее 50% от уровня 1990г. Конечно, нельзя считать решением финансовых проблем ученых области размер суммы выделяемой на ежегодные гранты губернатора в сфере науки и техники. На 25 грантов выделяется 1 млн. рублей. И эта более чем скромная сумма не меняется, несмотря на инфляционные процессы и пр., в течение последних 5 лет. В книге также рассматриваются вопросы взаимодействия с федеральными органами и организациями и в том числе с Российским фондом фундаментальных исследований, а также поддержки издательских проектов.
Серьезный анализ, сделанный авторами новой книги "Научный потенциал Тульской области", позволил выявить наиболее актуальных и работоспособных исследователей и изобретателей, которых в дальнейшем будут приглашать для выполнения научно- технических работ, использовать в качестве экспертов и консультантов, членов комиссий научно-технических и ученых советов. Важно и выявление предприятий, располагающих научным потенциалом, для последующего использования этой информации при выборе исполнителей научно-технических работ. Жаль, что в отличие от книги, изданной в 2000г., не сделан рейтинг авторов в зависимости от количества публикаций, патентов за исследуемый период. Вероятно, было бы крайне полезно дать материалы о работе научно-технических обществ (химического общества им. Менделеева, общества металлургов, энергетиков и др. общественных объединений СНИО). Дать, как и в издании 2000г., более подробное сравнение научного потенциала Тульской области с потенциалом соседних областей. Материал книги дает администрации области возможность проанализировать положение в научной сфере региона.
Разработан инструмент для многофакторного анализа направления научных исследований и разработок, масштабов и темпов их проведения, эффективности полученных результатов, их практической ценности для развития регионов.
Можно поздравить составителей книги, специалистов комитета по науке, сотрудников Тульской лаборатории информационных и математических исследований, Тульской областной универсальной библиотеки, правления СНИО, ВОИР и др. и всех нас с выходом столь нужного справочно-информационного издания. Ждем через 2-3 года нового справочника, участие в котором надеюсь примет большинство научных специалистов региона. Крайне полезно было бы издание подобных справочников и во всех регионах России. И в отличие от шутливого парадокса Сидни Смита ("Я сначала пишу предисловие, а книгу читаю потом, чтобы сохранить беспристрастность), я все же прочитал книгу и высказал, наверное, субъективные представления, но бесспорно о полезном материале, который, как говорится, будет лежать на письменных столах многих исследователей.
Литература
Профессор, доктор химических наук,Заслуженный химик России Голосман Е.З.
Пост-симпозиум "Каталитические методы использования возобновляемого сырья: топливо, энергия, химические продукты"
Институт катализа им. Г.К. Борескова Сибирского отделения РАН при поддержке Роснауки и содействии Генерального секретариата по исследованиям и технологиям министерства развития Греции провел XVII Международную конференцию по химическим реакторам (ХИМРЕАКТОР-17) и пост-симпозиум "Каталитические методы использования возобновляемого сырья: топливо, энергия, химические продукты" на базе Российского центра науки и культуры г. Афины 15-19 мая 2006 года. Эта конференция, совмещенная с выставкой по ресурсосберегающим технологиям и охране окружающей среды, является традиционной и проводится раз в два года в крупных научных, производственных и культурных центрах мира. Соорганизаторами конференции являются Европейская федерация по химической технологии и РОСЗАРУБЕЖЦЕНТР.
Конференция ХИМРЕАКТОР-17 проходила в отеле "Феникс" в Афинах, пост-симпозиум - на острове Крит. Общее число участников из 41 страны мира превысило 250 человек, они представили более 200 докладов. На конференцию приехали ученые из ведущих стран мира, таких как США, Германия, Великобритания, Голландия, Франция, Италия, Испания, Япония, а также из стран с быстро развивающейся в настоящее время экономикой (Китай, Сингапур, Колумбия, Саудовская Аравия) и многих других. Число российских участников конференции, пост-симпозиума и выставки составило более 100 человек, ими было сделано 35% всех научных сообщений.
Научная программа конференции ХИМРЕАКТОР-17 отражала следующие направления: кинетика каталитических реакций; физико-химические и математические основы процессов в реакторах; разработка каталитических процессов и реакторов: моделирование, оптимизация, применение новых и усовершенствованных катализаторов; каталитические методы производства топлива и энергии - производство водорода, экологически безопасных топлив, экологически безопасная энергетика. Программа пост-симпозиума была посвящена каталитическим технологиям производства топлива, энергии и химических продуктов из возобновляемого сырья.
 Тематика представленных на конференции докладов была традиционно широкой и затрагивала буквально все аспекты разработки, оптимизации и применения химических реакторов, по всей глубине спектра исследований - от фундаментальных основ осуществления реакционных процессов до вопросов их эксплуатации в промышленности. Также традиционно, основное внимание было уделено каталитическим технологиям и каталитическим реакторам.
Тематика представленных на конференции докладов была традиционно широкой и затрагивала буквально все аспекты разработки, оптимизации и применения химических реакторов, по всей глубине спектра исследований - от фундаментальных основ осуществления реакционных процессов до вопросов их эксплуатации в промышленности. Также традиционно, основное внимание было уделено каталитическим технологиям и каталитическим реакторам.
В области фундаментальных аспектов значительное внимание было уделено вопросам кинетики реакций и моделирования различных реакционных процессов. В частности, в пленарной лекции проф. Жильбера Фрома (Университет Техаса, США) была представлена теория "одиночных событий", которая за последние годы обеспечила существенный практический прорыв в области кинетики и моделирования сложных реакционных систем, в том числе - в процессах нефтепереработки и нефтехимии. Вопросам кинетики каталитических реакций тонкого синтеза сложных ассиметричных органических соединений была посвящена пленарная лекция проф. Дмитрия Мурзина (Университет Турку, Финляндия). Кроме этого, направление кинетики каталитических реакций было представлено большим числом устных и постерных докладов. В рамках тематики физико-химических и математических процессов в химических реакторах следует отметить ряд интересных докладов по моделированию аэродинамики течения реакционной смеси в химических реакторах.
В пленарной лекции проф. Жан-Клода Шарпентье (Лион, Франция) была предложена общая концепция разработки реакторов и реакционных процессов - от фундаментальных исследований реакций на молекулярном уровне до инжиниринга промышленных реакционных процессов. В частности, была показана высокая перспективность применения структурированных микрореакторов, которым также было уделено внимание в ряде других докладов.
На конференции были представлены разработки по новым перспективным типам химических реакторов. Среди этих разработок особый интерес представляют реактора с катализаторами на стекловолокнистых носителях, которым была посвящена пленарная лекция проф. Баира Бальжинимаева (Институт катализа СО РАН, Россия), а также ряд других выступлений. Эти реактора весьма перспективны и уже находят свое практическое применение в таких областях, как очистка олефинов от соединений ацетиленового ряда, окисление диоксида серы, селективное галогенирование углеводородов, очистка и утилизация газообразных и жидких промышленных отходов и выбросов. Также весьма немалый интерес представляют разработки по электропромотированным блочным катализаторам, которые были представлены в пленарной лекции проф. Костаса Вайенаса (Университет Патр, Греция).
Как и на предыдущих конференциях "Химреактор", существенное внимание было уделено вопросам осуществления химических реакций в искусственно создаваемых нестационарных условиях. Эти вопросы рассматривались в пленарной лекции проф. Иржи Ханика (Институт фундаментальных основ химических процессов, Прага, Чехия), посвященной нестационарным режимам в трехфазных реакторах. Значительное число докладов в этом разделе было посвящено перспективным нестационарным процессам селективного окисления углеводородов.
Вообще, вопросы разработки различных процессов селективного окисления сформировали один из наиболее значительных и интересных сегментов конференции. В пленарной лекции проф. Анжелики Лемонидоу (Технический университет г. Салоники, Греция) были представлены результаты разработки и исследования катализаторов окислительного дегидрирования пропана в пропилен. Этому процессу, а также ряду других селективных реакций были посвящены многие устные и стендовые доклады.
В целом, были представлены практически все актуальные направления применения химических реакторов, такие как нефтехимия и нефтепереработка, синтез ценных химических продуктов и полупродуктов, очистка выбросов и утилизация отходов и пр.
Однако, пожалуй, наибольшим вниманием пользовалась предметная область, связанная с вопросами производства и использования альтернативных топлив. В лекции проф. Валерия Кириллова (Институт катализа СО РАН, Россия) был представлен обзор современных достижений по процессам производства водорода для топливных элементов из различных видов углеводородного и органического сырья. Эта тематика в весьма широком спектре (производство водорода и синтез-газа, в том числе - за счет окислительной конверсии метана, синтез моторных топлив из природного газа и биологического возобновляемого сырья, энергетическое использование некондиционных топлив, в частности - низкоконцентрированных метановоздушных выбросов и др.) также была представлена в большом числе докладов на устных и стендовых сессиях конференции.
В целом, можно констатировать, что очередная конференция ХИМРЕАКТОР успешно отразила прогресс, достигнутый в мировой науке и промышленной практике в области разработки и исследования химических реакционных процессов за последние годы и позволила ее участникам получить ценную научную информацию, установить контакты для будущего сотрудничества, а также сформулировать наиболее актуальные направления будущих перспективных разработок и исследований.
Пост-симпозиум " Каталитические методы использования возобновляемого сырья: топливо, энергия, химические продукты" проходил сразу после основной конференции ХИМРЕАКТОР на живописном острове Крит в комфортабельном отеле "Нана".
В рамках данной конференции подобный пост-симпозиум проводился впервые. Актуальность его проведения в первую очередь заключается в необходимости интенсификации применения каталитических технологий в процессах переработки биомассы с целью оптимизации процессов получения целевых продуктов - биотоплива, углеродсодержащих материалов, органических соединений специального применения. При этом основная направленность пост-симпозиума заключалась в рассмотрении процессов переработки биомассы в энергетических целях: каталитического пиролиза и газификации биомассы с целью получения генераторного газа, синтез-газа, водорода, а также получения биодизельного топлива этерификацией растительных масел.
Двухдневная научная программа пост-симпозиума включала две пленарные лекции и девятнадцать устных сообщений. В целом тематики докладов можно условно разделить на несколько направлений:
Особенно необходимо отметить лекции по получению водорода, а именно доклады проф. Ланни Шмидта (Университет Миннесоты, США), проф. Панагиотиса Кечагиопулуса (Университет Аристотеля в Салониках, Греция), д-ра Норберта Васена (ETA, Италия), проф. Вячеслава Кафарова (Индустриальный университет Сантадера, Колумбия) и проф. Валерия Кириллова (Институт катализа СО РАН, Россия). Докладчики предложили различные способы получения биоводорода. Так, например, проф. Шмидт предложил перспективный способ получения водорода в микрореакторе через автотермический риформинг спиртов с временем контакта менее 10 мс в присутствии нанесенных Rh, Pt, Pd катализаторов, промотированных щелочными металлами. Другие авторы также предлагали получать водород через паровой риформинг жидкой бионефти, карбонизированных древесных пеллет, биоэтанола. Каждый из предложенных методов имеет очевидные преимущества и соответствующую перспективную область применения.
Новые способы получения биодизельного топлива из природных масел предложили проф. Айсе Акин (Университет Косаели, Турция), проф. Дмитрий Мурзин (Университет Турку, Финляндия) и проф. Луис Гандиа (Университет Наварры, Испания).
Первая группа авторов предлагает использовать в качестве катализаторов этерификации гетерогенные системы - липазу, иммобилизованную на гидроталькит или цеолиты. Группа проф. Луиса Гандиа (Университет Наварры, Испания) разрабатывает синтез биодизеля также в присутствии гетерогенных катализаторов - нанесенных на Al2O3 и SiO2 гидроксидов щелочных металлов. К сожалению, пока гетерогенные катализаторы нестабильны и обладают меньшей активностью, чем их гомогенные аналоги. Тем не менее, это направление имеет большую перспективу для промышленного применения в будущем.
Важную лекцию по процессам каталитической переработки растительной биомассы в России прочитал проф. Борис Кузнецов (Институт химии и химической технологии, Красноярск, Россия). Его презентация состояла из ряда примеров применения катализаторов при переработке биомассы, включая окислительную каталитическую деполимеризацию лигнина, каталитическую конверсию полисахаридов в ценные химикаты, а также каталитическую карбонизацию древесины и целлюлозы. Отмечено, что основной проблемой переработки древесины является до сих пор нерешенная проблема комплексной утилизации лигнина.
Кроме того, ряд лекций пост-симпозиума был посвящен каталитическому получению различных ценных органических соединений из продуктов первичной переработки биомассы. Например, проф. Дмитрий Мурзин предложил способ получения молочной кислоты из лактозы в присутствии катализаторов на основе золота. Д-р Ирина Симакова (Институт катализа СО РАН, Россия) доложила результаты исследования получения насыщенных кислот из растительного масла на гранулированном Pd/C. Аспирант Наталья Максимчук (Институт катализа СО РАН, Россия) разрабатывает метод селективного окисления a -пинена при использовании мягких окислителей (O2, H2O2) на Со-содержащих полиоксометалатах и NH2-модифицированных силикатных матрицах. Следует отметить активное участие молодых ученых из многих стран с интересными устными и стендовыми докладами как на конференции ХИМРЕАКТОР, так и на пост-симпозиуме.
В ходе конференции и на ее завершающей стадии состоялись Круглые столы по вопросам международного сотрудничества в области химической технологии. Участниками отмечено, что основное внимание на конференции и пост-симпозиуме было приковано к решению жизненно важных и особенно актуальных на сегодняшний день проблем энергетических ресурсов. При этом впервые акцент делался на каталитические методы решения энергетических проблем, особенно для производства энергии из возобновляемого сырья, процессов глубокой переработки природных ресурсов. Участие в работе конференции и пост-симпозиума ведущих специалистов мира в области химической технологии как из академических, так и промышленных структур обеспечило возможность обменяться опытом и наметить стратегию дальнейшего сотрудничества. Присутствие таких крупных ученых как профессор Жильбер Фрома (США), профессор Жан-Клод Шарпeнтье (Франция), профессор Ланни Шмидт (США), занимающих лидирующие позиции в мире по данному научному направлению, придало конференции и пост-симпозиуму особый статус, повысило их научный уровень. Сочетание работ исключительно глубокого фундаментального характера с докладами, имеющими важное практическое значение, стало особенностью прошедшего научного форума.
На фоне все возрастающего интереса к конференции ХИМРЕАКТОР особое значение приобретает усиление ее международного рейтинга и признание лидирующего статуса и серьезных позиций российской науки в области химической технологии и катализа. Опыт организации конференций ХИМРЕАКТОР показывает, что проведение научных мероприятий на базе Российских центров науки и культуры различных стран (две предыдущие конференции были проведены на базе Российских домов науки и культуры Хельсинки и Берлина) способствует развитию научно-технических связей, продвижению российских технологий на мировой рынок, укреплению экономических и культурных отношений между государствами. Целесообразность такого организационного решения признана и западными учеными, которые предлагают свои страны и содействие в организации для проведения следующей конференции ХИМРЕАКТОР. В качестве возможных вариантов на заключительном заседании рассматривались города Брюссель, Вена, Люксембург со стабильно работающими Российскими центрами науки и культуры. Но наиболее вероятным местом проведения следующей конференции ХИМРЕАКТОР-18 была признана Барселона (Испания). Участники конференции отметили, что она прошла на высоком научном и организационном уровне, им были созданы все условия для конструктивной работы.
 В рамках XII Международной конференции по химическим реакторам ХИМРЕАКТОР-17 и симпозиума по каталитическим процессам переработки возобновляемого сырья Институтом катализа им. Г.К. Борескова СО РАН по поручению Роснауки была организована выставка-семинар "Химические и каталитические технологии создания экологически безопасных производств". Выставка не случайно была приурочена к проводимой конференции - тематики мероприятий неразрывно связаны друг с другом, и их совместное проведение имеет уже сложившуюся традицию - в рамках Международной конференции ХИМРЕАКТОР-16 (Берлин, Германия, 2003 год) Министерством науки, промышленности и технологий была проведена выставка "Ресурсосберегающие технологии и охрана окружающей среды".
В рамках XII Международной конференции по химическим реакторам ХИМРЕАКТОР-17 и симпозиума по каталитическим процессам переработки возобновляемого сырья Институтом катализа им. Г.К. Борескова СО РАН по поручению Роснауки была организована выставка-семинар "Химические и каталитические технологии создания экологически безопасных производств". Выставка не случайно была приурочена к проводимой конференции - тематики мероприятий неразрывно связаны друг с другом, и их совместное проведение имеет уже сложившуюся традицию - в рамках Международной конференции ХИМРЕАКТОР-16 (Берлин, Германия, 2003 год) Министерством науки, промышленности и технологий была проведена выставка "Ресурсосберегающие технологии и охрана окружающей среды".
Организация выставки-семинара по каталитическим технологиям создания экологически безопасных производств имела целью ознакомление научных и деловых кругов Греции с последними достижениями в этой области российских организаций и предприятий и расширение прямых научных и коммерческих связей наших стран в этом направлении. Поскольку выставка проводилась в рамках конференции, собравшей более 250 участников из 41 страны мира, целью также являлось, и это очень важно, содействие продвижению предлагаемых разработок на западный рынок, демонстрация российского инвестиционного потенциала для многих зарубежных инвесторов.
Для демонстрации на выставке-семинаре были изготовлены следующие планшеты на английском языке по основным разработкам в области химических и каталитических технологий создания экологически безопасных производств :
В ходе проведенных мероприятий подписано соглашение о развитии сотрудничества с Греческим центром возобновляемых энергетических ресурсов. Регионы Юго-Восточной Европы являются крайне перспективными с точки зрения создания новейших каталитических технологий в области нефтехимии и альтернативных источников энергии, в том числе на базе возобновляемого сырья.
Принявшие участие в работе семинара специалисты отметили высокий уровень российских разработок и проявили заинтересованность в приобретении представленных на выставке технологий и материалов, выразили интерес в расширении прямых научных и коммерческих связей с российскими предприятиями и организациями.
Секретарь конференции Т.В. Замулина
К.х.н. А.Н. Загоруйко
К.х.н. В.А. Яковлев
Ultrafast electron microscopy
By integrating fast laser methods with transmission electron microscopy (TEM), researchers at Caltech have developed a microscopy technique that combines Angstrom spatial resolution with femtosecond time resolution (Proc. Natl. Acad. Sci. USA 2005, 102, 7069). The procedure, which was developed by Ahmed H. Zewail, Vladimir A. Lobastov and Ramesh Srinivasan, has been used to image various types of samples including gold crystals, amorphous carbon, and cells from rat intestines. Unlike conventional TEM, in which a hot cathode supplies electrons continuously via thermionic emission, in the Caltech method, electron emission is caused by illuminating the cathode with weak femtosecond laser pulses. Because it liberates very few electrons per pulse, the procedure sidesteps some of the difficulties that tend to broaden the electron beam and limit resolution. It also enables TEM images and videos to be recorded with unprecedented time resolution, thereby providing a route to atomic-scale dynamics studies of complex systems.
HTTP://WWW.CEN-ONLINE.ORG
С & EN / MAY 16, 2005
LG develops green route
 LG Chem has developed a nonchlorine process for making the chemical intermediate terephthaldehyde. Current terephthaldehyde plants, which are mostly in China and India, use chlorine as a reactant and yield, the coproduct hydrochloric acid, LG says. It expects the new process to lower the price of terephthaldehyde by 50%: or more. The South Korean company plans to license the technology and start operating its own plant in 2008.
LG Chem has developed a nonchlorine process for making the chemical intermediate terephthaldehyde. Current terephthaldehyde plants, which are mostly in China and India, use chlorine as a reactant and yield, the coproduct hydrochloric acid, LG says. It expects the new process to lower the price of terephthaldehyde by 50%: or more. The South Korean company plans to license the technology and start operating its own plant in 2008.
HTTP://WWW.CEN-ONLINE.ORG
С & EN /DECEMBER 5, 2005
Moving subsurface hydrogen atoms
Scientists at Pennsylvania State University have demonstrated a technique for imaging and manipulating hydrogen atoms just below the surface of a palladium crystal (Proc. Natl. Acad. Sci. USA 2005, 102,17907). Hydrogen interactions with precious metals are of key importance in hydrogen-storage applications, fuel cells, and other areas. In particular, subsurface H atoms have been fingered as intermediates in hydrogenation reactions on Pd, but until now the species has not been observed directly By treating a Pd crystal with hydrogen at elevated temperature and pressure, E. Charles H. Sykes, Paul S. Weiss, and coworkers prepare samples in which a small amount of H atoms are absorbed into the crystal’s bulk. .Then, by applying voltage pulses to select positions on the crystal surface with a scanning tunneling microscope tip, the team induces the atoms to accumulate at stable sites just below the top Pd layer, where they can form patterns of lines measuring just a few nanometers wide, as shown.
HTTP://WWW.CEN-ONLINE.ORG
С & EN / DECEMBER 12, 2005
Microwaves do enhance reaction rates
Microwave ovens have become popular tools to facilitate chemical reactions, but it has been unclear if the observed enhanced reaction rates result from rapid heating or from interactions of microwaves with the bonds of the reactants. Chemical engineer Roshan Jachuck of Clarkson University, Potsdam, N.Y., and his coworkers have now shown that microwave radiation indeed has a significant impact on reaction rates (Green Chem. 2006, 8, 29). Jachuck’s group designed and built a continuous capillary microreactor that can be maintained at a constant temperature while being irradiated with microwaves. The researchers used the reactor to study the iron-catalyzed conversion of benzyl alcohol to benzaldehyde. The reactor produced benzalde hyde in about 75% yield under optimized conditions, which equated to a reaction time of about 15 seconds and a flow rate of about 1 mL/minute. The key observation was that increasing the microwave intensity at constant temperature increased die yield of benzaldehyde. Jachuck envisions that frequency-tunable microwave microreactors could be used for lab- or pilot-scale projects or for production of low-volume active pharmaceutical ingredients.
HTTP://WWW.CEN-ONLINE.ORG
С & EN / JANUARY 30, 2006
Enzyme activity: Out with the old, in with the new
Mimicking the natural process of evolution, researchers have redesigned a natural enzyme, thereby causing it to lose its original activity and adopt a new one (Science 2006, 311, 535). Hak-Sung Kim of the Korea Advanced Institute of Science & Technology, Daejon, South Korea, and coworkers started with a core structure of the enzyme glyoxalase II that has thiolester hydrolysis activity. They subjected the protein to an approach called SIAFE (simultaneous incorporation and adjustment of functional elements) in conjunction with directed evolution (iterative modification and selection for desirable activity). The result was evMBL8, a designed enzyme with the ability to hydrolyze (b -lactam amide bonds, a type of activity on which bacterial resistance to (b-lactam antibiotics is based. Key to the change was the replacement of several of the enzyme’s surface loop structures. The researchers say they hope the technique can be extended to convert other structures into enzymes that catalyze diverse reactions, including some not found in nature.
HTTP://WWW.CEN-ONLINE.ORG
С & EN / JANUARY 30, 2006
Double C-F/C-H bond activation
 Superstrong C-F bonds don’t readily lend themselves to activation and subsequent reaction, but that hasn’t stopped chemists from edging closer to a general method for C-F activation. In one of the latest efforts, Kohei Fuchibe and Takahiko Akiyama of Gakushuin University, in Tokyo, report a "double activation" reaction in which a C-F bond and a C-H bond in close proximity in the same molecule are jointly activated, leading to ring-closing and formation of a new molecule (J. Am. Chem. Soc. 2006,128, 1434).
The researchers reacted o-phenyl-a,a,a
-trifluorotoluene with NbCl5 and LiAlH4 to form fluorene in up to 82% yield (shown). They also synthesized a series of substituted fluorenes from various substituted phenyltrifluorotoluenes. "This is a pretty cool reaction," notes Oleg V. Ozerov of Brandeis University, Waltham , Mass., who also works on C-F activation chemistry. "This chemistry goes beyond what anyone else has been able to accomplish in that C-F activation is coupled with C-H activation in a C-C bond-forming step," he says. The substituted fluorenes are potential core structures for pharmaceuticals and for polymers used in molecular electronics.
Superstrong C-F bonds don’t readily lend themselves to activation and subsequent reaction, but that hasn’t stopped chemists from edging closer to a general method for C-F activation. In one of the latest efforts, Kohei Fuchibe and Takahiko Akiyama of Gakushuin University, in Tokyo, report a "double activation" reaction in which a C-F bond and a C-H bond in close proximity in the same molecule are jointly activated, leading to ring-closing and formation of a new molecule (J. Am. Chem. Soc. 2006,128, 1434).
The researchers reacted o-phenyl-a,a,a
-trifluorotoluene with NbCl5 and LiAlH4 to form fluorene in up to 82% yield (shown). They also synthesized a series of substituted fluorenes from various substituted phenyltrifluorotoluenes. "This is a pretty cool reaction," notes Oleg V. Ozerov of Brandeis University, Waltham , Mass., who also works on C-F activation chemistry. "This chemistry goes beyond what anyone else has been able to accomplish in that C-F activation is coupled with C-H activation in a C-C bond-forming step," he says. The substituted fluorenes are potential core structures for pharmaceuticals and for polymers used in molecular electronics.
HTTP://WWW.CEN-ONLINE.ORG
С & EN / JANUARY 30, 2006
Уважаемые коллеги!
Приглашаем Вас принять участие в работе III Международной конференции "Катализ: теория и практика". Конференция будет проводиться в год 100-летия со дня рождения академика Г.К. Борескова в Новосибирском научном центре с 4 по 8 июля 2007 г.
Организационный комитет
Заявки на участие и доклад регистрируются в режиме on-line на веб-сайте конференции
Адрес Оргкомитета для переписки:
Старцевой Людмиле Яковлевне
Институт катализа СО РАН
просп. Акад. Лаврентьева,5, Новосибирск, 630090
Тел.\факс: (383)330-62-97
E-mail: star@catalysis.ru
| September 17- 22, 2006 4th European Summer School on Electrochemical Engineering Palic, Yugoslavia |
Velizar Stankovic Technical Faculty Bor Vojske Jugoslavije 12 Bor 19210 United Kingdom Tel.: +381 30 424 555 Fax: +381 30 421 078 E-mail: vstankovic@tf.bor.ac.yu Web site:http://www.shd.org.yu/ESSEE4/ |
September 18-22, 2006 3rd Mid-European Clay Conference (MECC-06) Opatija, Croatia |
E-mail: mecc06@gfz.hr Web site: http://mecc06.gfz.hr |
| Сентябрь 19-29, 2006 Международная школа-конференция "Космический вызов XXI века. Новые материалы и технологии для ракетно-космической техники" (SPACE'2006) Севастополь, Крым |
Акад. Берлин А.А. 119991 Москва, ул. Косыгина 4, ИХФ РАН Tel.: (495) 939 7267 Fax: (495) 651 2191 E-mail: space@chph.ras.ru Web site: http://www.chph.ras.ru/~space/Space2006rus.htm |
| September 23-26, 2006 5th International Conference on Inorganic Materials Ljubljana, Slovenia |
Web site: http://www.im-conference.elsevier.com |
September 25-27, 2006 7th International Conference on The Scale-Up of Chemical Processes Vilamoura, Portugal |
Claire Francis Scientific Update LLP Maycroft Place Stone Cross Mayfield, TN20 6EW United Kingdom Tel.: +44 (0) 1435 873062 Fax: +44 (0) 1435 872734 E-mail: claire@scientificupdate.co.uk Web site:http://www.scientificupdate.co.uk |
Сентябрь 25-Октябрь 1, 2006 Выставка по водоподготовке и очистке сточных вод (AQUATECH-2006) Амстердам, Нидерланды |
Родькина Ирина Симачева Марина ЗАО "Фирма СИБИКО Интернэшнл" (Компания ЭКВАТЭК) 107078 Москва, а/я 173 Tel.: (495) 782 10 13, 101 46 21 E-mail: office@sibico.com Web site: www.sibico.com, www.aquatechtrade.com |
September 26-29, 2006 8th International Conference on Fundamental and Applied Aspects of Physical Chemistry Belgrade, SCG |
Faculty of Physical Chemistry Studentski trg 12-16, P.O. Box 137 11001, Belgrade, SCG Tel./Fax: + 381 11 187 133 E-mail: dfh@ffh.bg.ac.yu Web site: http://www.iofh.bg.ac.yu/physchem2006/ |
Октябрь 2-6, 2006 Харьковская нанотехнологическая ассамблея: VII Международная конференция "Вакуумные технологии и оборудование" (ICVTE-7) & Международный научно-практический симпозиум "Наноструктурные функциональные покрытия для промышленности" (ISNFCI) XVIII Международный симпозиум “Тонкие пленки в оптике и наноэлектронике” (ISTFONE-18) Международный семинар “Вакуумно-дуговой разряд: физика, технологии и устройства” (ISVA) & II Школа молодых ученых и специалистов по нанотехнологиям, наноструктурным покрытиям и пленкам Харьков, Украина |
Шулаев Валерий Михайлович Редкокаша Александр Петрович Кириленко Анна Юрьевна 61108, Украина, Харьков, а/я 10363 Tel.: (057) 335 64 32, 335 63 23 Tel./Fax: (057) 335 25 45, 335 35 29 E-mail: v.shulayev@kipt.kharkov.ua Web site: www.ottom.com.ua |
October 2-6, 2006 Chemical Engineering for Scientists Baildon, United Kingdom |
Rachel Robinson Institution of Chemical Engineers 165-189 Railway Terrace Rugby CV21 3HQ, United Kingdom Tel.: +44 (0)1788 578214 Fax: +44 (0)1788 560833 E-mail: courses@icheme.org Web site: http://www.icheme.org/chemengsci |
| October 3-5, 2006 Catalytic Cross Coupling Reactions in Aromatic and Heteroaromatic Synthesis: Mechanism, Method & Strategy Nice, France |
Karen Thurley Scientific Update LLP Maycroft Place Stone Cross, Mayfield, TN20 6EW United Kingdom Tel.: +44 (0) 1435 873062 Fax: +44 (0) 1435 872734 E-mail: sciup@scientificupdate.co.uk Web site:http://www.scientificupdate.co.uk |
October 3-5, 2006 Chemical Development & Scale Up in the Fine Chemical Industry Amsterdam, Netherlands |
Claire Francis Scientific Update LLP Maycroft Place Stone Cross, Mayfield, TN20 6EW United Kingdom Tel.: +44 (0) 1435 873062 Fax: +44 (0) 1435 872734 E-mail: sciup@scientificupdate.co.uk Web site:http://www.scientificupdate.co.uk |
October 8-11, 2006 Organocatalysis Tutzing, Germany |
Andrea Kohl l DECHEMA e.V. Theodor-Heuss-Allee 25 Frankfurt am Main, 60486 Germany Tel.: +49-69-7564-235 Fax: +49-69-7564-441 E-mail: koehl@dechema.de Web site: http://events.dechema.de/tusy45 |
October 10-12, 2006 Asia Biofuels Conference & Expo IV Beijing, China |
Wendy Vincent Tel.: +01 605 323 0119 E-mail:wendy@biofuelsconferences.com Web site: www.asiabiofuels.com |
October 11-13, 2006 Advanced Polymers for Emerging Technologies Busan, Korea |
Prof. Sung Chul Kim Department of Chemical Engineering Korea Advanced Institute of Sci. & Tech. 373-1 Guseongdong, Yuseong-gu Daejeon 305-701. Korea Tel.: +82 42 869 3914 Fax: +82 42 869 8435 E-mail: kimsc@kaist.ac.kr Web site: www.psk30.org |
| October 13-14, 2006 9th International Conference on Laboratory Automation: Faster Process Development - New Tools for New Challenges Philadelphia, USA |
Claire Francis Scientific Update LLP Maycroft Place Stone Cross, Mayfield, TN20 6EW United Kingdom Tel.: +44 (0) 1435 873062 Fax: +44 (0) 1435 872734 E-mail: sciup@scientificupdate.co.uk Web site: http://www.scientificupdate.co.uk |
October 13-16, 2006 International Workshop "Dynamics of Correlated Particles in the Continuum" St.Petersburg, Russia |
Web site: http://www.ioffe.ru/ |
October 13-18, 2006 Reduced Nitrogen in Ecology and the Environment Obergurgl, Austria |
Jane Dutton European Science Foundation ESF Research Conferences 1 quai Lezay-Marnesia, Strasbourg 67080 France Tel.: +33 (0)388 76 71 35 Fax: +33 (0)388 36 69 87 E-mail: jdutton@esf.org Web site:http://www.esf.org/conferences/ c06203 |
October 16-20, 2006 27th Latin American Congress on Chemistry & 6th International Congress of Chemistry and Chemical Engineering Havana City, Cuba |
Prof. Alberto J. Nunez Selles Center of Pharmaceutical Chemistry Sociedad Cubana de Quimica Ave 21 & 200, Rpto. Atabey Apdo. 16042 Havana, CP 11600, Cubabr>Tel.: +53 7 218 178 Fax: +53 7 273 6471 E-mail: alberto@cgf.co.cu Web site: www.loseventos.cu/XXVIICLAQ/ |
October 17-18, 2006 Green Chemistry Basel, Switzerland |
Karen Thurley Scientific Update LLP Maycroft Place Stone Cross, Mayfield, TN20 6EW United Kingdom Tel.: +44 (0) 1435 873062 Fax: +44 (0) 1435 872734 E-mail: sciup@scientificupdate.co.uk Web site:http://www.scientificupdate.co.uk |
October 18-20, 2006 Chemical Development & Scale Up in the Fine Chemical Industry Durham, North Carolina, USA |
Claire Francis Scientific Update LLP Maycroft Place Stone Cross, Mayfield, TN20 6EW United Kingdom Tel.: +44 (0) 1435 873062 Fax: +44 (0) 1435 872734 E-mail: sciup@scientificupdate.co.uk Web site:http://www.scientificupdate.co.uk |
Октябрь 24-25, 2006 I Всероссийская научная конференция “Переработка углеводородного сырья. Комплексные решения” (Левинтеровские чтения) Самара, Россия |
Жилкина Евгения Олеговна Самарский государственный технический университет Химико-технологический факультет ГОУ ВПО Кафедра Химической технологии переработки нефти и газа ул. Молодогвардейская 244, Самара, 443100 Россия Tel./Fax: 846 442 35 80 Е-mail: levinter@sstu.smr.ru |
| October 24-27, 2006 Great Wall World Renewable Energy Forum (GWREF)& Exhibition (GWREF2006) Beijing,China |
Qin Haiyan General Secretary Chinese Wind Energy Association (CWEA) Beijing, China Tel:(+86) 13801 167 582 E-mail: qinhy@cgc.org.cn Web site: www.gwref.org |
October 25-27, 2006 International Conference on Modeling in Chemical and Biological Engineering Sciences Bangkok, Thailand |
Prof. Jumras Limtrakul Nanotechnology Center, Kasetsart University Research and Development Institute, Bangkok 10900, Thailand Tel.: 662 942 89 00 ext 304 E-mail: fsciirl@ku.ac.th Prof.Gregory Yablonsky Department of Chemical Engineering, Washington University, St. Louis, USA Tel.: 314 935 43 67 E-mail: gy@che.wustl.edu Web site: http://nanocenter.sci.ku.ac.th |
October 26-29, 2006 7th International Young Scientists Conference & International Conference "Optics and High Technology Material Science SPO 2006" Kyiv, Ukraine |
Viktor Lysiuk Taras Shevchenko National Univ. of Kyiv Department of Physics prospect Glushkova 2, bld.1 room 248, Kyiv 03022 Ukraine Tel.: +380 663 305 605 Fax: +380 445 264 507 E-mail: lysiuk@univ.kiev.ua Web site: http://www.spie.kiev.ua/spo |
October 29-November 10, 2006 NATO – ASI International school on "New Organic Chemistry Reactions and Methodologies for Green Production" Lecce – Otranto, Italy |
Vittorio Esposito INCA Lecce Italy Tel.: +39 0832 297231 Fax: +39 0832 297231 E-mail: incalecce@unile.it Web site:http://venus.unive.it/inca/education/na to-asi/index.php |
| October 30-November 3, 2006 Principles and Applications of Time-Resolved Fluorescence Spectroscopy Berlin, Germany |
PicoQuant GmbH Rudower Chaussee 29 (IGZ), Berlin12489 Germany Tel.: +49 30 6392 6560 Fax: +49 30 6392 6561 E-mail: trfcourse@pq.fta-berlin.de Web site:http://www.picoquant.com/_trfcourse.htm |
November 1-30, 2006 10th International Conference on Synthetic Organic Chemistry (Internet Conference) Switzerland |
Jullio A. Seijas Universidad of Santiago de Compostela. Campus Lugo Alfonso X el Sabio Facultad de Ciencias Lugo 27002 Spain Tel.: +34 982 285 900 ext. 24062 Fax: +34 982 285 872 E-mail: qoseijas@lugo.usc.es Web site: http://www.usc.es/congresos/ecsoc/10/ECSOC10.htm |
November 20-22, 2006 3rd Russian Conference “Physical Problems of Hydrogen Energy–2006” St.Petersburg, Russia |
Web site: http://www.ioffe.ru/HE2006/ |
November 2006 International Workshop “Thermoelectrics and their Applications” St.Petersburg, Russia |
Web site: http://www.ioffe.ru/ |
November 22-25, 2006 5th International Conference on Unsteady-State Processes in Catalysis (USPC-5) Suita City, Japan |
Prof. Takashi Aida USPC-5 Conference Secretariat Department of Chemical Engineering Tokyo Institute of Technology O-okayama Meguro-ku, Tokyo 152-8552, Japan Tel./Fax: +81 3 5734 2883 E-mail: uspc5@chemeng.titech.ac.jp Web site: http://www.chemeng.titech.ac.jp/~uspc5/ndex.html |
December 10-13, 2006 Conference and Exhibition on Catalysis in GCC Countries Kuwait |
Web site: www.gcccat.com |
December 11-15, 2006 3rd International Conference ElecMol'06 Grenoble, France |
Celine Deleval CEA-Grenoble UMR5819-SPrAM 17, Rue des Martyrs Grenoble Cedex9 38054 France Tel.: +33 4 3878 4001 Fax: +33 4 3878 5691 E-mail: info@elecmol.org Web site: http://www.elecmol.org |
| January 31-February 2, 2007 3rd International Symposium on the Separation and Characterization of Natural and Synthetic Macromolecules (SCM-3) Amsterdam, Netherlands |
Robert Smits Secretary of the Organizing Committee Roelsstraat 20 Oostduinkerke B-8670 Belgium Tel.: +32 58 523116 Fax: +32 58 514575 E-mail: scm@ordibo.be Web site: http://www.ordibo.be/ |
February 4-8, 2007 Australian Colloid and Interface Symposium Sydney, Australia |
Jane Yeaman Tulips Meetings Management PO Box 116 Salamander Bay NSW 2317 Australia Tel.: + 61 2 4984 2554 Fax: + 61 2 4984 2755 E-mail: acis@pco.com.au Web site: http://www.colloid-oz.org.au |
February 4-7, 2007 2nd North American Symposium on Chemical Reaction Engineering (NASCRE-2) Houston, Texas, USA |
Dan Luss University of Houston Bala Subramaniam University of Kansas Kurt VandenBussche UOP LLC ISCRE the International Symposia on Chemical Reaction Engineering, Inc. The JW Marriott at the Galleria 5150 Westheimer, USA Tel.: 1 713 961 1500 Web site:http://www.iscre.org/NASCRE2 |
February 7-8, 2007 International Workshop on Mathematics in Chemical and Biochemical Kinetics and Engineering (MACKiE-2) Houston, Texas, USA |
Prof. Gregory Yablonsky Department of Chemical Engineering Washington University in St. Louis Campus Box 1198 One Brookings Drive St. Louis 63130-4899, USA Tel.: 1 314 935 4367 E-mail: gy@che.wustl.edu David H. West Senior Scientist Epoxy R&D The Dow Chemical Company 2301 N. Brazosport Blvd.; B-1217 Freeport, TX 77541-3257, USA Tel.: 1 979 238 5140 E-mail: dwest@dow.com Web site:http://www.iscre.org/NASCRE2 |
February 11-16, 2007 3rd International Conference on Advanced Materials and Nanotechnology (AMN-3) Wellington, New Zealand |
Alison Downard University of Canterbury Ilam Rd, Christchurch New Zealand Tel.: +64 3 3642501 Fax: +64 3 3642110 E-mail:alison.downard@canterbury.ac.nz Web site:http://www.macdiarmid.ac.nz/AMN3 |
February 14-16, 2007 4th International Gas Analysis Symposium & Exhibition (GAS 2007) Rotterdam, Netherlands |
Web site: http://www.gas2007.org |
March 10-17, 2007 International Conference on Nanotechnology: Science and Application (NanoTech Insight 2007) Luxor, Egypt |
Dr. Mohamed M. S. Abdel-Mottaleb SabryCorp 18-El Amirallay Eid Mohamed Heliopolis-West Cairo11531 Egypt Tel.: +49 179 749 2510 Fax: +49 180 500 290 621 Web site: http://www.nanoinsight.net |
March 12-14, 2007 Water Status Monitoring under the WFD Lille, France |
Maggi Churchouse 3 East Barn Market Weston Road, Thelnetham Diss IP22 1JJ UK Tel.: +44 (0)1359 221 004 Fax: +44 (0)1359 221 004 E-mail: Maggi@WFDLille2007.org Web site:http://www.wfdlille2007.org |
April 2-4, 2007 Faraday Discussion 136: Crystal Growth and Nucleation London, UK |
Louise Drayton RSC Conferences Thomas Graham House Science Park, Milton Road Cambridge CB4 0WF UK Tel.: +44 (0) 1223 432 380 Fax: +44 (0) 1223 423 623 E-mail: conferences@rsc.org Web site: http://www.rsc.org/FD136 |
April 2-4, 2007 Nanoparticles: New Opportunities and Challenges for Colloid Scientists Coventry, UK |
Fiona Nalden RSC Conferences Thomas Graham House Science Park, Milton Road Cambridge CB4 0WF UK Tel.: +44 (0) 1223 432 380 Fax: +44 (0) 1223 423 623 E-mail: conferences@rsc.org Web site: http://www.rsc.org/NanoColloid07 |
April 3-6, 2007 World Congress on Radiochemistry & Nuclear Sciences (WorldCor 2007) Washington, D.C., USA |
Larry Burchfield The Radiochemistry Society P.O. Box 3091 Richland, WA 99354 USA E-mail:WorldCor@radiochemistry.org Web site:http://www.WorldCor.Radiochemistry.org |
April 15-21, 2007 17th International Conference on Phosphorus Chemistry Xiamen, China |
Prof. Yufen Zhao Xiamen University Department of Chemistry Xiamen, 361005 China Tel.: +86 5922 185 610 Fax: +86 5922 186 292 E-mail: yfzhao@xmu.edu.cn Web site: www.iupac.org |
April 22-24, 2007 1st UK-US Conference on Chemical and Biological Sensors and Detectors Bermuda |
Louise Drayton RSC Conferences Thomas Graham House Science Park, Milton Road Cambridge CB4 0WF UK Tel.: +44 (0) 1223 432254 Fax: +44 (0) 1223 423623 E-mail: conferences@rsc.org Web site: http://www.rsc.org/sensors07 |
April 26-27, 2007 Making and Using Fluoroorganic Molecules Florence, Italy |
Kate Laird Scientific Update LLP Maycroft Place Stone Cross Mayfield TN20 6EW UK Tel.: + 44 (0)1435 873 062 Fax: + 44 (0)1435 872 734 E-mail: scientificupdate.co.uk Web site:http://www.scientificupdate.co.uk |
May 7-11, 2007 15th European Biomass Conference & Exhibition “Biomass for Energy, Industry and Climate Protection - From Research to Market Deployment” Berlin, Germany |
Angela Grassi ETA-Renewable Energies Piazza Savonarola 10 50132 Florence, Italy Tel.: +39 055 500 2174 Direct line for Event Organisation Dept.: +39 055 500 2280 Fax : +39 055 573425 E-mail: eta.fi@etaflorence.it Web site: www.etaflorence.it Peter Helm WIP-Renewable Energies Sylvensteinstr. 2 81369 Munich, Germany Tel.: +49 89 720 12735 Fax: +49 89 720 12791 E-mail: wip@wip-munich.de Web site: www.wip-munich.de E-mail:biomass.conf@etaflorence.it Web site: www.conference-biomass.com |
May 16-19, 2007 3rd International Symposium of Federation of European Societies on Trace Elements and Minerals (FESTEM) Santiago de Compostela, Spain |
Bermejo-Barrera Pilar University of Santiago de Compostela Faculty of Chemistry Avenida de las Ciencias sn Santiago de Compostela 15782 Spain Tel.: + 346 0094 2346 Fax: + 349 8159 5012 E-mail: pbermejo@usc.es Web site:http://www.seqc.es/festemmeting/ |
May 22-24, 2007 12th International Symposium Loss Prevention and Safety Promotion in the Process Industries Edinburgh, UK |
Rosemary Cragg IChemE Davis Building165 - 189 Railway Terrace Rugby CV21 3HQ United Kingdom Tel.: + 44 (0) 1788 534476 Fax: + 44 (0) 1788 560833 E-mail: rcragg@icheme.org Web site: http://www.icheme.org /LossPrevention2007 |
June 6-10, 2007 8th International Symposium on Carbanion Chemistry (ISCC-8) University of Wisconsin, USA |
Kevin Jantzi Valparaiso University Neils Science Center 1610 Campus Drive East Valparaiso 46383 USA Tel.: + (219) 464 5201 Fax: + (219) 464 5489 E-mail: kevin.jantzi@valpo.edu Web site: http://www.chem.wisc.edu/iscc8/ |
Июнь 12-16, 2007 IX Международная Четаевская конференция “Аналитическая механика, устойчивость и управление движением” Иркутск – оз. Байкал, Россия |
Кононенко Галина Борисовна Tel.: (3952) 42 71 00 Fax: (3952) 51 16 16 E-mail: snv@icc.ru, chetayev_conference2007@icc.ru |
June 17-22, 2007 20th North American Catalysis Society Meeting Houston, TX, USA |
E-mail: nacs2007@lsu.edu Web site: http://www.20nam.org |
June 26-30, 2007 Modern Physical Chemistry for Advanced Materials Kharkiv, Ukraine |
Natalya Vodolazkaya School of Chemistry V.N.Karazin Kharkiv National University 4 Svoboda Square Kharkiv 61077 Ukraine Tel.: +38 057 707 5445 Fax: +38 057 705 1261 E-mail:izmailov2007@univer.kharkov.ua Web site: http://izmailov2007.univer.kharkov.ua |
Июль 1-6, 2007 XVI Международной конференции по химической термодинамике (RCCT-2007) & Конференция "Проблемы сольватации и комплексообразования в растворах" Суздаль, Россия |
Институт химии растворов 153045, г. Иваново, ул. Академическая, 1 E-mail: rcct2007@isc-ras.ru Web site: www.isc-ras.ru/RCCT2007/ |
July 2-4, 2007 Faraday Discussion 137: The Spectroscopy and Dynamics of Microparticles Bristol, United Kingdom |
Fiona Nalden RSC Conferences Thomas Graham House Science Park, Milton Road Cambridge CB4 0WF UK Tel.: +44 (0) 1223 432 254 Fax: +44 (0) 1223 423 623 E-mail: conferences@rsc.org Web site: http://www.rsc.org/FD137 |
July 4-6, 2007 11th European Meeting on Fire Retardant Polymers (FRPM07) Bolton, United Kingdom |
Nicky Wake Don't Panic Projects Ltd PO Box 448 Bury BL8 9AU UK Tel./Fax: + 44 (0)1706 828 855 E-mail: nicky@frpm07.com Web site: http://www.frpm07.com |
July 4-7, 2007 9th FIGIPAS-Meeting in Inorganic Chemistry Vienna, Austria |
Karl Kirchner Institute of Applied Synthetic Chemistry Getreidemarkt,9 Vienna A-1060 Austria Tel.: +43 1 58801 16301 Fax: +43 1 58801 16399 E-mail: figipas@tuwien.ac.at Web site: http://figipas.tuwien.ac.at |
July 6-8, 2007 1st Gordon-Kenan Graduate Research Seminar on Ogranometallic Chemistry Newport, Rhode Island, USA |
Nora Radu DuPont Co. Experimental Station PO Box 80328 Wilmington, DE 19880 USA Tel.: + 302 695 3363 Fax: + 302 695 8412 E-mail: nora.s.radu@usa.dupont.com Web site:http://www.grc.uri.edu/07sched.htm# GKSS |
July 7-14, 2007 Synthesis and Mechanism Queensland, Australia |
Curt Wentrup The University of Queensland Chemistry Building St Lucia Brisbane 4072 Australia Tel.: +61 7 3365 3817 Fax: +61 7 3365 4299 E-mail: wentrup@uq.edu.au Web site:http://earth.chemistry.uq.edu.au/heron4/ |
July 8-12, 2007 Greenhouse Gases: Mitigation and Utilization Kingston, Canada |
Philip Jessop Queen's University Department of Chemistry 90 Bader Lane Kingston K7L 3N6 Canada Tel.: + 1 613 533 3212 Fax: + 1 613 533 6669 E-mail: jessop@chem.queensu.ca Web site:http://www.chem.queensu.ca/greenho |
July 8-12, 2007 Nanostructured Polymers and Polymer Nanocomposites Prague, Czech Republic |
Libor Matějka Institute of Macromolecular Chemistry Academy of Sciences of the Czech Republic Heyrovskйho nбm. 2 Praha 6 CZ 162 06 Czech Republic Tel.: +420 296 809 332 Fax: +420 296 809 410 E-mail: sympo@imc.cas.cz Web site: http://www.imc.cas.cz/sympo/46micros.html |
July 8-13, 2007 ESOC 2007 Conference Dublin, Ireland |
Paulene McKeever ESOC 2007 Secretariat C/o Conference Organisers Ltd Clifton House, Lr Fitzwilliam Street Dublin 2 Ireland Tel.: +353 1 662 0125 Fax: +353 1 662 0126 E-mail: info@conferenceorganisers.ie Web site: http://www.esoc2007.com |
July 11-13, 2007 Modern Synthetic Methods - Reaction to Reality & Chiral USA Philadelphia, USA |
Kate Laird Scientific Update LLP Maycroft Place Stone Cross Mayfield TN20 6EW UK Tel.: + 44 (0)1435 873 062 Fax: + 44 (0)1435 872 734 E-mail: sciup@scientificupdate.co.uk Web site:http://www.scientificupdate.co.uk |
July 16-18, 2007 Analytical Research Forum 2007 Glasgow, UK |
Ruth Needham RSC Conferences Thomas Graham House Science Park, Milton Road Cambridge CB4 0WF UK Tel.: +44 (0)1223 432 380 Fax: +44 (0)1223 423 623 E-mail: conferences@rsc.org Web site: http://www.rsc.org/ARF07 |
July 16-20, 2007 13th International Symposium on Relations between Homogeneous and Heterogeneous Catalysis (ISHHC XIII) Berkeley, California, USA |
Prof. Gabor A. Somorjai Department of Chemistry University of California D58 Hildebrand #1460 Berkeley, CA 94720 Tel.: (510) 642 4053 Fax: (510) 643 9668 E-mail: somorjai@socrates.berkeley.edu, ishhc13@berkeley.edu, ishhc13@calmail.berkeley.edu Web site: http://www.cchem.berkeley.edu/~gas. grp http://www.lbl.gov/Conferences/ISHH C/XIII/ |
July 16-20, 2007 30th International Conference on Solution Chemistry Perth, Australia |
Prof. Glenn Hefter School of Mathematical and Physical Sciences Murdoch University Murdoch, WA 6150 Australia Tel.: +61 8 9360 2226 Fax: +61 8 9360 1711 E-mail: g.hefter@murdoch.edu.au Web site:http://www.iupac.org/symposia/2007.html |
July 22-27, 2007 12th International Symposium on Novel Aromatic Compounds (ISNA-12) Tsuna-Gun, Japan |
Prof. Yoshito Tobe Division of Frontier Materials Science Osaka University Toyonaka, Osaka University, Japan Tel.: +81 6 6850 6225 Fax: +81 6 6850 6229 E-mail: tobe@chem.es.osaka-u.ac.jp Web site:ww.iupac.org/symposia, www.chem.es.osaka-u.ac.jp/ISNA12 |
July 23-25, 2007 2nd International Conference on Semiconductor Photochemistry Schoolhill, Aberdeen, UK |
Peter Robertson Robert Gordon University Schoolhill, Aberdeen AB10 1FR UK Tel.: +44 (0)1224 262 01 Fax: +44(0)1224 262 16 E-mail: sp-2@rgu.ac.uk Web site:http://www.rgu.ac.uk/cree/sp-2 |
August 2-6, 2007 14th International Symposium on Organometallic Chemistry Directed towards Organic Synthesis (OMCOS-14) Nara, Japan |
Prof. Kazuhiko Takai Department of Applied Chemistry Okayama University Faculty of Engineering Tsuchimanaka 3-1-1, Okayama 700-8530, Japan Tel.: +81 86 251 8097 Fax: +81 86 251 8094 E-mail: ktakai@cc.okayama-u.ac.jp Web site: www.iupac.org/symposia |
August 4-12, 2007 IUPAC 44th General Assembly Turin, Italy |
IUPAC Secretariat Tel.: +1 919 485 8700 Fax: +1 919 485 8706 E-mail: secretariat@iupac.org Web site: www.iupac.org |
August 5-11, 2007 IUPAC 41st Congress Turin, Italy |
IUPAC Secretariat Tel.: +1 919 485 8700 Fax: +1 919 485 8706 E-mail: IUPAC2007@unito.it Web site: www.iupac.org/symposia/congress07.html , www.iupac.org |
August 12-16, 2007 8th International Conference on Heteroatom Chemistry (ICHAC-8) Riverside, CA, USA |
Chris Reed UC Riverside Center for S and P Block Chemistry Riverside, California 92521 USA Tel.: + 1(951) 827 5197 Fax: + 1(951) 827 2027 E-mail: chris.reed@ucr.edu Web site: http://ichac-8.ucr.edu/ |
August 26-31, 2007 EUROPACAT VIII “From Theory to Industrial Practice” Turku/Abo, Finland |
Web site: www.abo.fi/europacat8 |
August 27-29, 2007 Discrete Element Methods-07 Brisbane, Australia |
Barry Wills Minerals Engineering International 18 Dracaena Avenue Falmouth, Cornwall TR11 2EQ UK Tel.: +44 (0)7768 234121 Fax: +44 (0)1326 318352 E-mail: bwills@min-eng.com Web site: http://www.min-eng.com/dem07/index.html |
September 3-5, 2007 Faraday Discussion 138: Nanoalloys - From Theory to Applications Birmingham, UK |
Fiona Nalden RSC Conferences Thomas Graham House Science Park, Milton Road Cambridge CB4 0WF UK Tel.: +44 (0) 1223 432254 Fax: +44 (0) 1223 423623 E-mail: conferences@rsc.org Web site: http://www.rsc.org/ConferencesAndE vents/RSCConferences/FD138/index.a sp |
September 6-9, 2007 2nd International Symposium Advanced micro- and mesoporous materials Varna, Bulgaria |
Georgi Vayssilov Faculty of Chemistry, University of Sofia Blvd. J. Bourchier 1 Sofia 1126 Bulgaria Tel.: + (359 2) 8161 338 Fax: + (359 2) 9625 438 E-mail: micro2007@innoslab.com Web site:http://micro2007.innoslab.com |
September 9-12, 2007 11th International Conference on Chemistry and the Environment “Chemistry, Environment and Human Activity in Civilization Development” Torun, Poland |
Boguslaw Buszewski Nicolaus Copernicus University Department of Environmental Chemistry 7 Gagarin Str, Torun 87-100 Poland Tel.: +45 56 611 4308 Fax: +45 56 654 2477 E-mail: analityk@chem.uni.torun.pl Web site:http://www.chem.uni.torun.pl/en/ |
September 9-14, 2007 EUROanalysis XIV Antwerp, Belgium |
E-mail: koen.janssens@ua.ac.be Web site: http://www.euroanalysisxiv.ua.ac.be/ |
September 16-20, 2007 8th Simposium on Catalysis Applied to Fine Chemical (8th CAFC) Verbania Pallanza, Italy |
E-mail: n.ravasio@istm.cnr.it |
October 12-18, 2007 FACSS 2007 Memphis, TN, USA |
FACSS P O Box 24379 Santa Fe NM 87502 USA Tel.: + 1 505 820 1648 Fax: + 1 505 989 1073 E-mail: facss@facss.org Web site: http://www.facss.org |
November 5-6, 2007 Process Systems in the Metallurgical Industry 07 Cape Town, South Africa |
Barry Wills Minerals Engineering International 18 Dracaena Avenue Falmouth Cornwall TR11 2EQ UK Tel.: +44 7768 234121 Fax: +44 1326 318352 E-mail: bwills@min-eng.com Web site: http://www.min-eng.com/processsystems07/index.html |
November 14-15, 2007 3rd International Symposium on Solid-Liquid Separation (SLS 07) Cape Town, South Africa |
Barry Wills Minerals Engineering International 18 Dracaena Avenue Falmouth Cornwall TR11 2EQ UK Tel.: +44 7768 234121 Fax: +44 1326 318352 E-mail: bwills@min-eng.com Web site: http://www.min-eng.com/sls07/index.html |
January 28-30, 2008 10th International Symposium on Advances in Extraction Techniques (ExTech-2008) Brugge, Belgium |
Robert Smits KVCV Roelsstraat 20 Oostduinkerke B-8670 Belgium Tel.: +32 58 523116 Fax: +32 58 514575 E-mail: robert.smits@kvcv.be Web site: http://www.ordibo.be |
January 30-February 1, 2008 10th International Symposium on Hyphenated Techniques in Chromatography and Hyphenated Chromatographic Analyzers (HTC-10) Bruges, Belgium |
Robert Smits Royal Flemish Chemical Society Roelsstraat 20 Oostduinkerke B-8670 Belgium Tel.: +32 58 523116 Fax: +32 58 514575 E-mail: robert.smits@kvcv.be Web site: http://www.ordibo.be |
June 15-19, 2008 World Hydrogen Energy Conference Queensland, Australia |
Jacki Brown ICMS Pty Ltd PO Box 3496 South Brisbane Queensland 4101 Australia Tel.: +61 7 3844 1138 Fax: +61 7 3844 0909 E-mail: whec2008@icms.com.au Web site: http://www.whec2008.com |
August 31- September 3, 2008 5th International Conference on Environment Catalysis ICEC-5 Belfast, UK |
E-mail: r.burch@qub.ac.uk Web site: www.centacat.qub.ac.uk/5icec |
September 1-2, 2008 2nd International FEZA School on Zeolites and September, 2-6, 2008 4th International FEZA Conference ”Advances in Nanoporous Materials” Paris, France |
Pr. Antoine Gedeon FEZA 2008 UPVC, laboratoire SIEN 4 place Jussieu, case 196 75252 Paris cedex 05, France Tel.: 331 44 27 5529 Fax: 331 44 27 5536 E-mail: ag@ccr.jussieu.fr E-mail: feza2009@upmc.fr Web site: http://www.sien.jussieu.fr |
September 16-20, 2008 2nd European Chemistry Congress Torino, Italy |
Evelyn McEwan EuCheMS Secretariat c/o RSC Burlington House, Piccadilly London W1J 0BA United Kingdom Tel.: +44 (0) 20 7440 3303 Fax: +44 (0) 20 7437 8883 E-mail: mcewane@rsc.org Web site: http://www.euchems.org |


